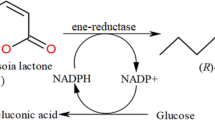
Abstract
Enzymes are able to perform reactions under mild conditions, e.g., pH and temperature, with remarkable chemo-, regio-, and stereoselectivity. Due to this feature the number of biocatalysts used in organic synthesis has rapidly increased during the last decades, especially for the production of chiral compounds. The present review highlights biotechnological processes for the production of chiral alcohols by reducing prochiral ketones with whole cells. Microbial transformations feature different characteristics in comparison to isolated enzymes. Enzymes that are used in whole-cell biotransformations are often more stable due to the presence of their natural environment inside the cell. Because reductase-catalyzed reactions are dependent on cofactors, one major task in process development is to provide an effective method for regeneration of the consumed cofactors. Many whole-cell biocatalysts offer their internal cofactor regeneration that can be used by adding cosubstrates, glucose or, in the case of cyanobacteria, simply light. In this paper, various processes carried out on laboratory and industrial scales are presented. Thereby, attention is turned to process parameters, e.g., conversion, yield, enantiomeric excess, and process strategies, e.g., the application of biphasic systems. The biocatalytic production of chiral alcohols utilizing isolated enzymes is presented in part I of this review (Goldberg et al., Appl Microbiol Biotechnol, 2007).





Similar content being viewed by others
References
Amidjojo M, Weuster-Botz D (2005) Asymmetric synthesis of the chiral synthon ethyl (S)-4-chloro-3-hydroxybutanoate using Lactobacillus kefir. Tetrahedron Asymmetry 16:899–901
Amidjojo M, Franco-Lara E, Nowak A, Link H, Weuster-Botz D (2005) Asymmetric synthesis of tert-butyl (3R,5S) 6-chloro-dihydroxyhexanoate with Lactobacillus kefir. Appl Microbiol Biotechnol 69:9–15
Bertau M, Burli M (2000) Enantioselective microbial reduction with baker’s yeast on an industrial scale. Chimia 54:503–507
Blacklock TJ, Sohar P, Butcher JW, Lamanec T, Grabowski EJJ (1993) An enantioselective synthesis of the topically-active carbonic anhydrase inhibitor MK-0507: 5,6-dihydro-(S)-4-(ethylamino)-(S)-6-methyl-4H-thieno[ 2,3-b]thiopyran-2-sulfonamide 7,7-dioxide hydrochloride. J Org Chem 58:1672–1679
Breuer M, Ditrich K, Habicher T, Hauer B, Keßeler M, Stürmer R, Zelinski T (2004) Industrielle Verfahren zur Herstellung von optisch aktiven Zwischenprodukten. Angew Chem 116:806–843, Angew Chem Int Ed 43:788–824
Chartrain M, Roberge C, Chung J, McNamara J, Zhao D, Olewinski R, Hunt G, Salmon P, Roush D, Yamazaki S, Wang T, Grabowski E, Buckland B, Greasham R (1999) Asymmetric bioreduction of (2-(4-nitro-phenyl)-N-(2-oxo-2-pyridin-3-yl-ethyl)-acetamide) to its corresponding (R)-alcohol [(R)-N-(2-hydroxy-2-pyridin-3-yl-ethyl)-2-(4-nitro-phenyl)-acetamide] by using Candida sorbophila MY 1833. Enzyme Microb Technol 25:489–496
Csuk R (1991) Baker’s yeast mediated transformations in organic chemistry. Chem Rev 91:49–97
Edegger K, Stampfer W, Seisser B, Faber K, Mayer SF, Oehrlein R, Hafner A, Kroutil W (2006a) Regio- and stereoselective reduction of diketones and oxidation of diols by biocatalytic hydrogen transfer. Eur J Org Chem 2006(8):1904–1909
Edegger K, Gruber CC, Faber K, Hafner A, Kroutil W (2006b) Optimization of reaction parameters and cultivation conditions for biocatalytic hydrogen transfer employing overexpressed ADH-′A′ from Rhodococcus ruber DSM 44541 in Escherichia coli. Eng Life Sci 6:149–154
Engelking H, Pfaller R, Wich G, Weuster-Botz D (2006) Reaction engineering studies on β-ketoester reductions with whole cells of recombinant Saccharomyces cerevisiae. Enzyme Microb Technol 38:536–544
Ernst M, Kaup B, Müller M, Bringer-Meyer S, Sahm H (2005) Enantioselective reduction of carbonyl compounds by whole-cell biotransformation, combining a formate dehydrogenase and a (R)-specific alcohol dehydrogenase. Appl Microbiol Biotechnol 66:629–634
Faber K (2004) Biotransformations in organic chemistry, 5th edn. Springer, Berlin Heidelberg New York
Floyd DM, Moquin RV, Atwal KS, Ahmed SZ, Spergel SH, Gougoutas JZ, Malley MF (1990) Synthesis of benzazepinone and 3-methylbenzothiazepinone analogues of diltiazem. J Org Chem 55:5572–5579
Goldberg K, Edegger K, Kroutil W, Liese A (2006) Overcoming the thermodynamic limitation in asymmetric hydrogen transfer reactions catalyzed by whole cells. Biotechnol Bioeng 95:192–198
Goldberg K, Schroer K, Lütz S, Liese A (2007) Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part I: processes with isolated enzymes. Appl Microbiol Biotechnol (in this issue)
Gröger H, Chamouleau F, Orologas N, Rollmann C, Drauz K, Hummel W, Weckbecker A, May O (2006) Enantioselektive Ketonreduktion mit “Designerzellen” bei hohen Substratkonzentrationen: hocheffizienter Zugang zu funktionalisierten optisch aktiven Alkoholen. Angew Chem 118:5806–5809, Angew Chem Int Ed 45:5677–5681
Haberland J, Kriegesmann A, Wolfram E, Hummel W, Liese A (2002a) Diastereoselective synthesis of optically active (2R,5R)-hexanediol. Appl Microbiol Biotechnol 58:595–599
Haberland J, Hummel W, Daußmann T, Liese A (2002b) New continuous production process for enantiopure (2R,5R)-hexanediol. Org Process Res Dev 6:458–462
Havel J, Weuster-Botz D (2006) Comparative study of cyanobacteria as biocatalysts for the asymmetric synthesis of chiral building blocks. Eng Life Sci 6:175–179
Holt RA (1996) Microbial asymmetric reduction in the synthesis of a drug intermediate. Chim Oggi 14:17–20
Jones TK, Mohan JJ, Xavier LC, Blacklock TJ, Mathre DJ, Sohar P, Turner Jones ET, Reamer RA, Roberts FE, Grabowski EJJ (1991) An asymmetric synthesis of MK-0417. Observations on oxazaborolidine-catalyzed reductions. J Org Chem 56:763–769
Kaluzna IA, Feske BD, Wittayanan W, Ghiviriga I, Stewart JD (2005) Stereoselective, biocatalytic reductions of α-chloro-β-keto esters. J Org Chem 70:342–345
Kaup B, Bringer-Meyer S, Sahm H (2004) Metabolic engineering of Escherichia coli: construction of an efficient biocatalyst for d-mannitol formation in a whole-cell biotransformation. Appl Microbiol Biotechnol 64:333–339
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka·M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Nakamura K, Matsuda T (2002) Reduction of ketones. In: Drauz K, Waldmann H (eds) Enzyme catalysis in organic synthesis, vol. III, 2nd edn. Wiley–VCH Verlag GmbH, Weinheim, pp 991–1047
Nakamura K, Yamanaka R, Tohi K, Hamada H (2000) Cyanobacterium-catalyzed asymmetric reduction of ketones. Tetrahedron Lett 41:6799–6802
Nanduri VB, Hanson RL, Goswami A, Wasylyk JM, LaPorte TL, Katipally K, Chung HJ, Patel RN (2001) Biochemical approaches to the synthesis of ethyl 5-(S)-hydroxyhexanoate and 5-(S)-hydroxyhexanenitrile. Enzyme Microb Technol 28:632–636
Patel RN, Robison RS, Szarka LJ, Kloss J, Thottathil JH, Muller RH (1991) Stereospecific microbial reduction of 4,5-dihydro-4-(4-methoxyphenyl)-6-(trifluoromethyl-1H-1)-benzazepin-2-one. Enzyme Microb Technol 13:906–912
Patel RN, McNamee CG, Banerjee A, Howell JM, Robison RS, Szarka LJ (1992) Stereoselective reduction of β-keto esters by Geotrichum candidum. Enzyme Microb Technol 14:731–738
Patel RN, Chu L, Chidambaram R, Zhu J, Kant J (2002) Enantioselective microbial reduction of 2-oxo-2-(1′,2′,3′,4′-tetrahydro-1′,1′,4′,4′-tetramethyl-6′-naphthalenyl)acetic acid and its ethyl ester. Tetrahedron Asymmetry 13:349–355
Peters J, Zelinski T, Kula MR (1992) Studies on the distribution and regulation of microbial keto ester reductases. Appl Microbiol Biotechnol 38:334–340
Rodriguez S, Kayser M, Stewart JD (1999) Improving the stereoselectivity of bakers’ yeast reductions by genetic engineering. Org Lett 1:1153–1155
Rodriguez S, Kayser MM, Stewart JD (2001) Highly stereoselective reagents for β-keto ester reductions by genetic engineering of baker’s yeast. J Am Chem Soc 123:1547–1555
Shaw NM, Robins KT, Kiener A (2003) Lonza: 20 years of biotransformations. Adv Synth Catal 345:425–435
Shih TL, Candelore MR, Cascieri MA, Chiu SHL, Colwell LF, Jr, Deng L, Feeney WP, Forrest MJ, Hom GJ, Maclntyre DE, Miller RR, Stearns RA, Strader CD, Tota L, Wyvratt MJ, Fisher MH, Weber AE (1999) L-770,644: a potent and selective human β3 adrenergic receptor agonist with improved oral bioavailability. Bioorg Med Chem Lett 9:1251–1254
Stampfer W, Kosjek B, Moitzi C, Kroutil W, Faber K (2002) Biocatalytic asymmetric hydrogen transfer. Angew Chem 114:1056–1059, Angew Chem Int Ed 41:1014–1017
Stampfer W, Edegger K, Kosjek B, Faber K, Kroutil W (2004) Simple biocatalytic access to enantiopure (S)-1-heteroarylethanols employing a microbial hydrogen transfer reaction. Adv Synth Catal 346:57–62
Stewart JD (2001) Dehydrogenases and transaminases in asymmetric synthesis. Curr Opin Chem Biol 5:120–129
Tan AWI, Fischbach M, Huebner H, Buchholz R, Hummel W, Daußmann T, Wandrey C, Liese A (2006) Synthesis of enantiopure (5R)-hydroxyhexane-2-one with immobilized whole cells of Lactobacillus kefiri. Appl Microbiol Biotechnol 71:289–293
Watanabe T, Koller K, Messner K (1998) Copper-dependent depolymerization of lignin in the presence of fungal metabolite, pyridine. J Biotechnol 62:221–230
Weckbecker A, Hummel W (2004) Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+-dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol Lett 26:1739–1744
Zaks A, Dodds DR (1997) Application of biocatalysis and biotransformations to the synthesis of pharmaceuticals. Drug Discov Today 2:513–531
Zhang J, Witholt B, Lia Z (2006) Efficient NADPH recycling in enantioselective bioreduction of a ketone with permeabilized cells of a microorganism containing a ketoreductase and a glucose 6-phosphate dehydrogenase. Adv Synth Catal 348:429–433
Acknowledgment
Both Goldberg and Schroer did equally contribute to this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldberg, K., Schroer, K., Lütz, S. et al. Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part II: whole-cell reductions. Appl Microbiol Biotechnol 76, 249–255 (2007). https://doi.org/10.1007/s00253-007-1005-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1005-x