Abstract
Superoxide dismutase (SOD) activity is one major defense line against oxidative stress for all of the aerobic organisms, and industrial production of this enzyme is highly demanded. The Cu/Zn superoxide dismutase gene (KmSOD1) of Kluyveromyces marxianus L3 was cloned and characterized. The deduced KmSod1p protein shares 86% and 71% of identity with Kluyveromyces lactis and Saccharomyces cerevisiae Sod1p, respectively. The characteristic motifs and the amino acid residues involved in coordinating copper and zinc and in enzymatic function were conserved. To the aim of developing a microbial production of Cu/Zn superoxide dismutase, we engineered the K. marxianus L3 strain with the multicopy plasmid YG-KmSOD1 harboring the KmSOD1 gene. The production of KmSOD1p in K. marxianus L3 and K. marxianus L3 (pYG-KmSOD1) in response to different compositions of the culture medium was evaluated. The highest specific activity (472 USOD mgprot −1) and the highest volumetric yield (8.8 × 105 USOD l−1) were obtained by the recombinant strain overexpressing KmSOD1 in the presence of Cu2+ and Zn2+ supplements to the culture media. The best performing culture conditions were positively applied to a laboratory scale fed-batch process reaching a volumetric yield of 1.4 × 106 USOD l−1.





Similar content being viewed by others
References
Bender JP, Mazutti MA, de Oliveira D, Di Luccio M, Treichel H (2006) Inulinase production by Kluyveromyces marxianus NRRL Y-7571 using solid state fermentation. Appl Biochem Biotechnol 129–132:951–958
Butt TR, Sternberg EJ, Gorman JA, Clark P, Hamer D, Rosenberg M, Crooke ST (1984) Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A 81:3332–3336
Campanella JJ, Bitincka L, Smalley J (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4:29
Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC (2004) Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci U S A 101:5964–5969
Chen XJ, Saliola M, Falcone C, Bianchi MM, Fukuhara H (1986) Sequence organization of the circular plasmid pKD1 from the yeast Kluyveromyces drosophilarum. Nucleic Acids Res 14:4471–4481
Corvo ML, Jorge JC, van't HR, Cruz ME, Crommelin DJ, Storm G (2002) Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochim Biophys Acta 1564:227–236
Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD (1997) The copper chaperone for superoxide dismutase. J Biol Chem 272:23469–23472
Dancis A, Haile D, Yuan DS, Klausner RD (1994) The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269:25660–25667
Dellomonaco C, Amaretti A, Zanoni S, Pompei A, Matteuzzi D, Rossi M (2007) Fermentative production of superoxide dismutase with Kluyveromyces marxianus. J Ind Microbiol Biotechnol 34:27–34
Emerit J, Samuel D, Pavio N (2006) Cu-Zn super oxide dismutase as a potential antifibrotic drug for hepatitis C related fibrosis. Biomed Pharmacother 60:1–4
Field LS, Luk E, Culotta VC (2002) Copper chaperones: personal escorts for metal ions. J Bioenerg Biomembr 34:373–379
Fonseca GG, Gombert AK, Heinzle E, Wittmann C (2007) Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res 7:422–435
Fridovich I (1978) The biology of oxygen radicals. Science 201:875–880
Fridovich I (1998) Oxygen toxicity: a radical explanation. J Exp Biol 201:1203–1209
Glerum DM, Shtanko A, Tzagoloff A (1996) Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J Biol Chem 271:14504–14509
Goulielmos GN, Arhontaki K, Eliopoulos E, Tserpistali K, Tsakas S, Loukas M (2003) Drosophila Cu,Zn superoxide dismutase gene confers resistance to paraquat in Escherichia coli. Biochem Biophys Res Commun 308:433–438
Harris N, Bachler M, Costa V, Mollapour M, Moradas-Ferreira P, Piper PW (2005) Overexpressed Sod1p acts either to reduce or to increase the lifespans and stress resistance of yeast, depending on whether it is Cu(2+)-deficient or an active Cu,Zn-superoxide dismutase. Aging Cell 4:41–52
Hart PJ, Balbirnie MM, Ogihara NL, Nersissian AM, Weiss MS, Valentine JS, Eisenberg D (1999) A structure-based mechanism for copper-zinc superoxide dismutase. Biochemistry 38:2167–2178
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Limtong S, Sringiew C, Yongmanitchai W (2007) Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour Technol 98:3367–3374
Lin SJ, Culotta VC (1995) The ATX1 gene of Saccharomyces cerevisiae encodes a small metal homeostasis factor that protects cells against reactive oxygen toxicity. Proc Natl Acad Sci U S A 92:3784–3788
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC (2005) Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J Biol Chem 280:22715–22720
Masoud W, Jespersen L (2006) Pectin degrading enzymes in yeasts involved in fermentation of Coffea arabica in East Africa. Int J Food Microbiol 110:291–296
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Moradas-Ferreira P, Costa V, Piper P, Mager W (1996) The molecular defences against reactive oxygen species in yeast. Mol Microbiol 19:651–658
Nishikawa M, Nagatomi H, Nishijima M, Ohira G, Chang BJ, Sato E, Inoue M (2001) Targeting superoxide dismutase to renal proximal tubule cells inhibits nephrotoxicity of cisplatin and increases the survival of cancer-bearing mice. Cancer Lett 171:133–138
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805–808
Saliola M, Mazzoni C, Solimando N, Crisa A, Falcone C, Jung G, Fleer R (1999) Use of the KlADH4 promoter for ethanol-dependent production of recombinant human serum albumin in Kluyveromyces lactis. Appl Environ Microbiol 65:53–60
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular Cloning: a Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sherman F, Fink GR, Hicks JB (1986) Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC (2001) A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem 276:38084–38089
Taylor WR (1986) The classification of amino acid conservation. J Theor Biol 119:205–218
Vorauer-Uhl K, Furnschlief E, Wagner A, Ferko B, Katinger H (2001) Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. Eur J Pharm Sci 14:63–67
Wesolowsky-Louvel M, Breunig KD, Fukuhara H (1996) Kluyveromyces lactis. In: Wolf K (ed) Nonconventional Yeasts in Biotechnology. Springer, Berlin, pp 139–201
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25:434–456
Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, Hashida M (2001) Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther 298:894–899
Yoo HY, Kim SS, Rho HM (1999) Overexpression and simple purification of human superoxide dismutase (SOD1) in yeast and its resistance to oxidative stress. J Biotechnol 68:29–35
Yu P (2007) A new approach to the production of the recombinant SOD protein by methylotrophic Pichia pastoris. Appl Microbiol Biotech 74:93–98
Yunoki M, Kawauchi M, Ukita N, Sugiura T, Ohmoto T (2003) Effects of lecithinized superoxide dismutase on neuronal cell loss in CA3 hippocampus after traumatic brain injury in rats. Surg Neurol 59:156–160
Zhang Y, Wang JZ, Wu YJ, Li WG (2002) Anti-inflammatory effect of recombinant human superoxide dismutase in rats and mice and its mechanism. Acta Pharmacol Sin 23:439–444
Acknowledgments
This study was partially supported by PRIN 2006 research program: “Yeast as sources of biodiversity for the production of molecules of agro-alimentary and pharmaceutical interest” and by Ateneo research funding La Sapienza 2007.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Fig. 1
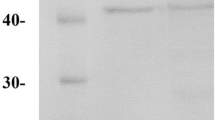
Coomassie-stained electrophoresis gel of the protein extracts utilized in the Western blotting against Sod1p reported in Fig. 2. The molecular mass of Sod1p is reported (GIF 1.62 mb)
Supplementary Fig. 1
Coomassie-stained electrophoresis gel of the protein extracts utilized in the Western blotting against Sod1p reported in Fig. 2. The molecular mass of Sod1p is reported (GIF 1.62 mb)
Rights and permissions
About this article
Cite this article
Raimondi, S., Uccelletti, D., Matteuzzi, D. et al. Characterization of the superoxide dismutase SOD1 gene of Kluyveromyces marxianus L3 and improved production of SOD activity. Appl Microbiol Biotechnol 77, 1269–1277 (2008). https://doi.org/10.1007/s00253-007-1270-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1270-8