Abstract
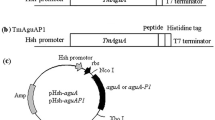
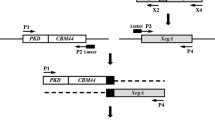
The flexible peptides (GGGGS)n (n ≤ 3), the α-helical peptides (EAAAK)n (n ≤ 3) and two other peptides were used as linkers to construct bifunctional fusions of β-glucanase (Glu) and xylanase (Xyl) for improved catalytic efficiencies of both moieties. Eight Glu-Xyl fusion enzymes constructed with different linkers were all expressed as the proteins of ca. 46 kDa in Escherichia coli BL21 and displayed the activities of both β-glucanase and xylanase. Compared to all the characterized fusions with the parental enzymes, the catalytic efficiencies of the Glu and Xyl moieties were equivalent to 304–426% and 82–143% of the parental ones, respectively. The peptide linker (GGGGS)2 resulted in the best fusion, whose catalytic efficiency had a net increase of 326% for the Glu and of 43% for the Xyl. The two moieties of a fusion with the linker (EAAAK)3 also showed net increases of 262 and 31% in catalytic efficiency. Our results highlight, for the first time, the enhanced bifunctional activities of the Glu-Xyl fusion enzyme by optimizing the peptide linkers to separate the two moieties at a reasonable distance for beneficial interaction.




Similar content being viewed by others
References
Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T (2001) Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng 14:529–532
Arai R, Wriggers W, Nishikawa Y, Nagamune T, Fujisawa T (2004) Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins 57:829–838
Bai Y, Shen WC (2006) Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm Res 23:2116–2121
Blazyk JL, Lippard SJ (2004) Domain engineering of the reductase component of soluble methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem 279:5630–5640
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang HC, Kaiser CM, Hartl FU, Barral JM (2005) De novo folding of GFP fusion proteins: high efficiency in eukaryotes but not in bacteria. J Mol Biol 353:397–409
Crasto CJ, Feng J (2000) LINKER: a program to generate linker sequences for fusion proteins. Protein Eng 13:309–312
Dien BS, Cotta MA, Jeffries TW (2003) Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol 63:258–266
Doi N, Yanagawa H (1999) Insertional gene fusion technology. FEBS Letters 457:1–4
Flint HJ, Martin J, McPherson CA, Daniel AS, Zhang JX (1993) A bifunctional enzyme, with separate xylanase and β(1, 3-1, 4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens. J Bacteriol 175:2943–2951
Gray NK, Hentze MW (1994) Regulation of protein synthesis by mRNA structure. Mol Biol Rep 19:195–200
Gustavsson M, Lehtio J, Denman S, Teeri TT, Hult K, Martinelle M (2001) Stable linker peptides for a cellulose-binding domain-lipase fusion protein expression in Pichia pastoris. Protein Eng 14:711–715
Gutman GA, Hatfield GW (1989) Nonrandom utilization of codon pairs in Escherichia coli. Proc Natl Acad Sci USA 86:3699–3703
Hoedemaeker FJ, Signorelli T, Johns K, Kuntz DA, Rose DR (1997) A single chain Fv fragment of p-glycoprotein-specific monoclonal antibody C219 design, expression, and crystal structure at 2.4 Å resolution. J Biol Chem 272:29784–29789
Hong SY, Lee JS, Cho KM, Math RK, Kim YH, Hong SJ, Cho YU, Kim H, Yun HD (2006) Assembling a novel bifunctional cellulose-xylanase from Thermotoga maritima by end-to-end fusion. Biotechnol Lett 28:1857–1862
Irwin B, Heck JD, Hatfield GW (1995) Codon pair utilization biases influence translational. J Biol Chem, 270:22801–22806
Karnielya S, Rayznera A, Sass E, Pines O (2006) a-Complementation as a probe for dual localization of mitochondrial proteins. Exp Cell Res 312:3835–3846
Levasseur A, Navarro D, Punt PJ, Belaich JP, Asther M, Record E (2005) Construction of engineered bifunctional enzymes and their overproduction in Aspergillus niger for improved enzymatic tools to degrade agricultural by-products. Appl Environ Microbiol 71:8132–8140
Lois AC, Barbara AS, John RM, Janet EP, Sharon LA, Andrew B, Glenn FP, Houston LL (1998) Targeting tumor cells via EGF receptors: selective toxicity of an HBEGF-toxin fusion protein. Int J Cancer 78:106–111
Longland AC, Theodorou MK, Sanderson R, Lister SJ, Powell CJ, Morris P (1995) Non-starch polysaccharide composition and in vitro fermentability of tropical forage legumes varying in phenolic content. Anim Feed Sci Technol 55:161–177
Lu P, Feng MG, Li WF, Hu CX (2006) Construction and characterization of a bifunctional fusion enzyme of Bacillus-sourced β-glucanase and xylanase expressed in Escherichia coli. FEMS Microbiol Lett 261:224–230
Maeda Y, Ueda H, Kazami J, Kawano G, Suzuki E, Nagamune T (1997) Engineering of functional chimeric protein G-Vargula luciferase. Anal Biochem 249:147–152
Mathlouthi N, Saulnier L, Quemener B, Larbier M (2002) Xylanase, b-glucanase, and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and b-glucanase used alone or in combination. J Agric Food Chem 50:5121–5127
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moura G, Pinheiro M, Silva R, Miranda I, Afreixo V, Dias G, Freitas A, Oliveira JL, Santos MAS (2005) Comparative context analysis of codon pairs on an ORFeome scale. Genome Biol 6:R28
Petriz J, Gottesman MM, Aran JM (2004) An MDR-EGFP gene fusion allows for direct cellular localization, function and stability assessment of p-glycoprotein. Curr Drug Deliv 1:43–56
Morag E, Bayer EA, Lamed R (1990) Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol 172:6098–6105
Remy I, Wilson IA, Stephen WM (1999) Erythropoietin receptor activation by a ligand-induced conformation change. Science 283:990–993
Rosenblum MG, Cheung LH, Liu Y, Marks JW (2003) Design, expression, purification, and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res 63:3995–4002
Salobir J (1998) Effect of xylanase alone and in combination with β-glucanase on energy utilization, nutrient utilization and intestinal viscosity of broilers fed diets based on two wheat samples. Arch Gefluegelkund 62:209–213
Seo HS, Koo YJ, Lim JY, Song JT, Kim CH, Kim JK, Lee JS, Choi YD (2000) Characterization of a bifunctional enzyme fusion of trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase of Escherichia coli. Appl Environ Microbiol 66:2484–2490
Shan D, Press OW, Tsu TT, Hayden MS, Ledbetter JA (1999) Characterization of scFv-Ig constructs generated from theAnti-CD20 mAb 1F5 using linker peptides of varying lengths. J Immunol 162:6589–6595
Trinh R, Gurbaxani B, Morrison SL, Seyfzadeh M (2004) Optimization of codon pair use within the (GGGGS)3 linker sequence results in enhanced protein expression. Mol Immunol 40:717–722
Trujillo M, Duncan R, Santi DV (1997) Construction of a homodimeric dihydrofolate reductase-thymidylate synthase bifunctional enzyme. Protein Eng 10:567–573
Volkel T, Korn T, Bach M, Muller R, Kontermann RE (2001) Optimized linker sequences for the expression of monomeric and dimeric bispecific single-chain diabodies. Protein Eng 14:815–823
Wang WWS, Das D, McQuarrie SA, Suresh MR (2007) Design of a bifunctional fusion protein for ovarian cancer drug delivery: single-chain anti-CA125 core-streptavidin fusion protein. Eur J Pharm Biopharm 65:398–405
Werner S, Marillonnet S, Hause G, Klimyuk V, Gleba Y (2006) Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proc Natl Acad Sci USA 103:17678–17683
Xue GP, Goblus KS, Orpin CG (1992) A novel polysaccharide hydrolase cNAD (celD) from Neocallimastix pareiciarum encoding three multi-functional catalytic domains with high endoglucanase, cellobiohydrolase and xylanase activities. J Gen Microbiol 138:2397–2403
Xue F, Gu Z, Feng J (2004) LINKER: a web server to generate peptide sequences with extended conformation. Nucleic Acids Res 32:562–565
Acknowledgements
Chang HC (Department of Cellular Biochemistry, Max Planck Institute of Biochemistry, Martinsried, Germany) is thanked for providing the nucleotide sequences of α-helical peptide linkers. This study was supported jointly by the Ministry of Science and Technology of China (2007DFA3100), the Natural Science Foundation of China (30571250), the Ministry of Education of China (IRT0535), and Zhejiang R&D Program (2007C12035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, P., Feng, MG. Bifunctional enhancement of a β-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl Microbiol Biotechnol 79, 579–587 (2008). https://doi.org/10.1007/s00253-008-1468-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1468-4