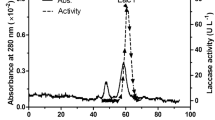
Abstract
The lack of a commercially available robust and inexpensive laccase is a major barrier to the widespread application of this enzyme in various industrial sectors. By using an efficient system developed in Streptomyces lividans, we have produced by homologous expression 350 mg L−1 of a bacterial laccase with a high purity and without any extensive purification. This is the highest production yield reported in the literature for a bacterial laccase. The secreted enzyme achieved oxidation under a wide pH range depending on the substrate: 4.0 for 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) and 9.0 for 2,6-dimethoxyphenol. Furthermore, this bacterial laccase was found to be quite resistant under various conditions. It withstands pH from 3.0 to 9.0, shows a great thermostability at 70°C and was highly resistant toward conventional inhibitors. For instance, while the laccase of Trametes versicolor was completely inhibited by 1 mM NaN3, the laccase of Streptomyces coelicolor was fully active under the same conditions. To assess application potential of this laccase, we have investigated its ability to decolourise Indigo carmine. This enzyme was able to rapidly decolourise the dye in the presence of syringaldehyde as a redox mediator.





Similar content being viewed by others
References
Alexandre G, Zhulin IB (2000) Laccases are widespread in bacteria. Trends Biotech 18:41–42
Alves A, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JGH, Wosten HAB (2004) Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol 70:6379–6384
Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C (2002) Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry 41:7325–7333
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Burton SG (2003) Laccases and phenol oxidases in organic synthesis—a review. Curr Org Chem 7:1317–1331
Camarero S, Ibarra D, Martinez MJ, Martinez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Chefetz B, Chen Y, Hadar Y (1998) Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl Environ Microbiol 64:3175–3179
Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2,2’’-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J 145:93–103
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Endo K, Hayashi Y, Hibi T, Hosono K, Beppu T, Ueda K (2003) Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J Biochem (Tokyo) 133:671–677
Enguita FJ, Martins LO, Henriques AO, Carrondo MA (2003) Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J Biol Chem 278:19416–19425
Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148:2159–2169
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol 9:601–605
Hullo MF, Moszer I, Danchin A, Martin-Verstraete I (2001) CotA of Bacillus subtilis is a copper-dependent laccase. J Bacteriol 183:5426–5430
Hurtubise Y, Shareck F, Kluepfel D, Morosoli R (1995) A cellulase/xylanase-negative mutant of Streptomyces lividans 1326 defective in cellobiose and xylobiose uptake is mutated in a gene encoding a protein homologous to ATP-binding proteins. Mol Microbiol 17:367–377
Jaouani A, Guillen F, Penninckx MJ, Martinez AT, Martinez MJ (2005) Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzyme Microb Technol 36:478–486
Kenealy WR, Jeffries TW (2003) Enzyme processes for pulp and paper: a review of recent developments. In: Goodell B, Nicholas DD, Schultz TP (eds) Wood deterioration and preservation: advances in our changing world, ACS Symposium Series 845, Washington, pp 210–239
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Kluepfel D, Vats-Mehta S, Aumont F, Shareck F, Morosoli R (1990) Purification and characterization of a new xylanase (xylanase B) produced by Streptomyces lividans 66. Biochem J 267:45–50
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Lomascolo A, Record E, Herpoel-Gimbert I, Delattre M, Robert JL, Georis J, Dauvrin T, Sigoillot JC, Asther M (2003) Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J Appl Microbiol 94:618–624
Machczynski MC, Vijgenboom E, Samyn B, Canters GW (2004) Characterization of SLAC: a small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci 13:2388–2397
Madzak C, Mimmi MC, Caminade E, Brault A, Baumberger S, Briozzo P, Mougin C, Jolivalt C (2006) Shifting the optimal pH of activity for a laccase from the fungus Trametes versicolor by structure-based mutagenesis. Protein Eng Des Sel 19:77–84
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Minussi RC, Pastore GM, Duran N (2007) Laccase induction in fungi and laccase/N–OH mediator systems applied in paper mill effluent. Bioresour Technol 98:158–164
Moldes D, Sanroman MA (2006) Amelioration of the ability to decolorize dyes by laccase: relationship between redox mediators and laccase isoenzymes in Trametes versicolor. World J Microbiol Biotechnol 22:1197–1204
Muñoz C, Guillén F, Martínez AT, Martínez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Perie FH, Reddy GVB, Blackburn NJ, Gold MH (1998) Purification and characterization of laccases from the white-rot basidiomycete Dichomitus squalens. Arch Biochem Biophys 353:349–355
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-A resolution containing a full complement of coppers. J Biol Chem 277:37663–37669
Rodriguez Couto S, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Sharma P, Goel R, Capalash N (2007) Bacterial laccases. World J Microbiol Biotechnol 23:823–832
Slomczynski D, Nakas JP, Tanenbaum SW (1995) Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol 61:907–912
Suzuki T, Endo K, Ito M, Tsujibo H, Miyamoto K, Inamori Y (2003) A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci Biotechnol Biochem 67:2167–2175
Ward AC (1992) Rapid analysis of yeast transformants using colony-PCR. BioTechniques 13:350
Yoshida H (1883) Chemistry of lacquer (urushi). J Chem Soc Trans 43:472–486
Acknowledgements
This work was supported by the Canada research chair on value-added papers from the Centre Intégré en Pâtes et Papiers (Trois-Rivières, Canada), AgroTerra Biotech (Trois-Rivières, Canada), the Institut National de la Recherche Scientifique—Institut Armand-Frappier (Laval, Canada) and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubé, E., Shareck, F., Hurtubise, Y. et al. Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl Microbiol Biotechnol 79, 597–603 (2008). https://doi.org/10.1007/s00253-008-1475-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1475-5