Abstract
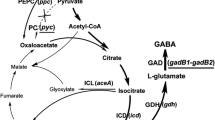
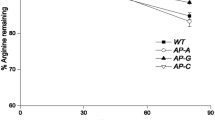
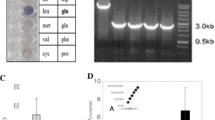
Corynebacterium glutamicum played a central role in the establishment of fermentative production of amino acids, and it is a model for genetic and physiological studies. The general aromatic amino acid transporter, AroPCg, was the sole functionally identified aromatic amino acid transporter from C. glutamicum. In this study, the ncgl1108 (named as pheP Cg), which is located upstream of the genetic cluster (ncgl1110 ∼ ncgl1113) for resorcinol catabolism, was identified as a new l-Phe specific transporter from C. glutamicum RES167. The disruption of pheP Cg resulted in RES167∆ncgl1108, and this mutant showed decreased growth on l-Phe (as nitrogen source) but not on l-Tyr or l-Trp. Uptake assays with unlabeled and 14C-labeled l-Phe and l-Tyr indicated that the mutants RES167∆ncgl1108 showed significant reduction in l-Phe uptake than RES167. Expression of pheP Cg in RES167∆ncgl1108/pGXKZ1 or RES167∆(ncgl1108-aroP Cg)/pGXKZ1 restored their ability to uptake for l-Phe and growth on l-Phe. The uptake of l-Phe was not inhibited by nine amino acids but by l-Tyr. The K m and V max values of RES167∆(ncgl1108-aroP Cg)/pGXKZ1 for l-Phe were determined to be 10.4 ± 1.5 μM and 1.2 ± 0.1 nmol min−1 (mg DW)−1, respectively, which are different from K m and V max values of RES167∆(ncgl1108-aroP Cg) for l-Phe [4.0 ± 0.4 μM and 0.6 ± 0.1 nmol min−1 (mg DW)−1]. In conclusion, this PhePCg is a new l-Phe transporter in C. glutamicum.





Similar content being viewed by others
References
Brown KD (1970) Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol 104(1):177–188
Burkovski A (ed) (2008) Corynebacteria: genomics and molecular biology. Caister Academic Press, Norfolk
Chaudhry MT, Huang Y, Shen XH, Poetsch A, Jiang CY, Liu SJ (2007) Genome-wide investigation of aromatic acid transporters in Corynebacterium glutamicum. Microbiology 153(3):857–865
Chye ML, Guest JR, Pittard J (1986) Cloning of the aroP gene and identification of its product in Escherichia coli K-12. J Bacteriol 167(2):749–753
Cosgriff AJ, Brasier G, Pi J, Dogovski C, Sarsero JP, Pittard AJ (2000) A study of AroP-PheP chimeric proteins and identification of a residue involved in tryptophan transport. J Bacteriol 182(8):2207–2217
D'Ambrosio SM, Glover GI, Nelson SO, Jensen RA (1973) Specificity of the tyrosine-phenylalanine transport system in Bacillus subtilis. J Bacteriol 115(2):673–681
DeBusk BG, DeBusk AG (1965) Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta 104(1):139–150
Eggeling L, Bott M (eds) (2005) Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton
Feng J, Che Y, Milse J, Yin YJ, Liu L, Ruckert C, Shen XH, Qi SW, Kalinowski J, Liu SJ (2006) The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J Biol Chem 281(16):10778–10785
Honore N, Cole ST (1990) Nucleotide sequence of the aroP gene encoding the general aromatic amino acid transport protein of Escherichia coli K-12: homology with yeast transport proteins. Nucleic Acids Res 18(3):653
Huang Y, Zhao KX, Shen XH, Chaudhry MT, Jiang CY, Liu SJ (2006) Genetic characterization of the resorcinol catabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72(11):7238–7245
Ikeda M, Katsumata R (1994) Transport of aromatic amino acids and its influence on overproduction of the amino acids in Corynebacterium glutamicum. J Ferment Bioeng 78(6):420–425
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62(2):99–109
Jack DL, Paulsen IT, Saier MH (2000) The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 146(8):1797–1814
Jakoby M, Ngouoto-Nkili C-E, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Techniq 13(6):437–441
Jetten MS, Follettie MT, Sinskey AJ (1994) Metabolic engineering of Corynebacterium glutamicum. Ann NY Acad Sci 721:12–29
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Möckel B, Pfefferle W, Pühler A, Rey DA, Rückert C, Rupp O, Sahm H, Wendisch VF, Wiegräbe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104(1–3):5–25
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175(17):5595–5603
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation. I. Production of L-glutamic acid by various microorganisms. J Gen Appl Microbiol 7:193–205
Konopka A (1993) Isolation and characterization of a subsurface bacterium that degrades aniline and methylanilines. FEMS Microbiol Lett 111(1):93–99
Koyanagi T, Katayama T, Suzuki H, Kumagai H (2004) Identification of the LIV-I/LS system as the third phenylalanine transporter in Escherichia coli K-12. J Bacteriol 186(2):343–350
Krings E, Krumbach K, Bathe B, Kelle R, Wendisch VF, Sahm H, Eggeling L (2006) Characterization of myo-inositol utilization by Corynebacterium glutamicum: the stimulon, identification of transporters, and influence on L-lysine formation. J Bacteriol 188(23):8054–8061
Lee SY, Kim YH, Min J (2010) Conversion of phenol to glutamate and proline in Corynebacterium glutamicum is regulated by transcriptional regulator ArgR. Appl Microbiol Biotechnol 85(3):713–720
Liao MK, Gort S, Maloy S (1997) A cryptic proline permease in Salmonella typhimurium. Microbiology 143(9):2903–2911
Marin K, Krämer R (2007) Amino acid transport systems in biotechnologically relevant bacteria. Microbiology Monographs 5:289–325. doi:https://doi.org/10.1007/7171_2006_069
Pi J, Wookey PJ, Pittard AJ (1991) Cloning and sequencing of the pheP gene, which encodes the phenylalanine-specific transport system of Escherichia coli. J Bacteriol 173(12):3622–3629
Qi SW, Chaudhry MT, Zhang Y, Meng B, Huang Y, Zhao KX, Poetsch A, Jiang CY, Liu S, Liu SJ (2007) Comparative proteomes of Corynebacterium glutamicum grown on aromatic compounds revealed novel proteins involved in aromatic degradation and a clear link between aromatic catabolism and gluconeogenesis via fructose-1,6-bisphosphatase. Proteomics 7(20):3775–3787
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Shen XH, Liu ZP, Liu SJ (2004) Functional identification of the gene locus ncg12319 and characterization of catechol 1,2-dioxygenase in Corynebacterium glutamicum. Biotechnol Lett 26(7):575–580
Shen X-H, Huang Y, Liu S-J (2005) Genomic analysis and identification of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Microbes Environ 20(3):160–167
Simic P, Sahm H, Eggeling L (2001) L-threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J Bacteriol 183(18):5317–5324
Tauch A, Kassing F, Kalinowski J, Puhler A (1995) The Corynebacterium xerosis composite transposon Tn5432 consists of two identical insertion sequences, designated IS1249, flanking the erythromycin resistance gene ermCX. Plasmid 34(2):119–131
Tauch A, Kirchner O, Loffler B, Gotker S, Puhler A, Kalinowski J (2002) Efficient electrotransformation of corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr Microbiol 45(5):362–367
Wehrmann A, Morakkabati S, Kramer R, Sahm H, Eggeling L (1995) Functional analysis of sequences adjacent to dapE of Corynebacterium glutamicum reveals the presence of aroP, which encodes the aromatic amino acid transporter. J Bacteriol 177(20):5991–5993
Xu Y, Yan DZ, Zhou NY (2006) Heterologous expression and localization of gentisate transporter Ncg12922 from Corynebacterium glutamicum ATCC 13032. Biochem Biophys Res Commun 346(2):555–561
Yang Z, Lv Y, Liao H, Xu K (2003) Multi wavelength RP-HPLC determination of the aromatic amino acids in compound amino acid injection. Chin J Pharmaceutical Analysis 23(6):2
Zhao KX, Huang Y, Chen X, Wang NX, Liu SJ (2010) PcaO positively regulates pcaHG of the beta-ketoadipate pathway in Corynebacterium glutamicum. J Bacteriol 192(6):1565–1572
Acknowledgements
We thank Dr. Ying Xu and Dr. Song-He Wang at Wuhan Institute of Virology, Chinese Academy of Sciences, for encouraging discussions and technical support of this work. This work was supported by grants (30730002 and 30725001) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, Z., Ding, JY., Li, T. et al. The ncgl1108 (PheP Cg) gene encodes a new l-Phe transporter in Corynebacterium glutamicum . Appl Microbiol Biotechnol 90, 2005–2013 (2011). https://doi.org/10.1007/s00253-011-3245-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3245-z