Abstract
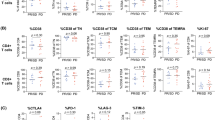
CD8+ T cells in the circulation of patients with head and neck cancer (HNC) were previously shown to be significantly more sensitive to, and preferentially targeted for, apoptosis than CD4+ T cells (Hoffmann et al., Clin Cancer Res, 8:2553–2562, 2002). To distinguish global from CD8+ subset-specific apoptosis, we studied Annexin-binding to naïve, memory, and effector subsets of CD8+ cells by multicolor flow cytometry. Age-related changes in naïve and effector CD8+ cell subsets were observed in patients and normal controls (NC). The frequencies of naïve (CD28+CD45RO-) CD8+ T cells were lower and those of memory (CD28+CD45RO+) and effector (CD28-) CD8+ T cells significantly higher in the circulation of HNC patients relative to age-matched NC. Among CD8+ T cells, the CD28- effector cell subset contained the highest proportion of Annexin-binding cells, while the naïve CD28+CD45RO- subset contained the lowest. This suggested a high turnover rate of the CD8+CD28- effector cell subset in patients with HNC, which was being compensated by a rapid transition of naïve CD8+ T cells to the effector cell pool. Following tumor resection, the frequency of CD8+CD28- T cells normalized in the patients, an indication that the presence of tumor had an influence on the size of CD8+CD28- T-cell pool. Ex vivo, in mixed lymphocyte-tumor cultures (MLTC) with semiallogeneic T cells as responders, CD8+CD28- T cells could be generated from CD8+CD28+ cells by repeated stimulations with tumor cells. These CD8+CD28- effector cells lysed the tumor, produced IFN-γ in response to the tumor, and strongly expressed granzyme B. Thus, the high rate of their apoptosis in the circulation of patients with HNC might be expected to contribute to tumor progression. However, the ex vivo generation of this cell subset was suppressed by strong CD28/B7 ligation or by overexpresson of MHC molecules on tumor cells, suggesting that adequate costimulation is necessary for protection from apoptosis. It appears that interactions of immune and tumor cells might determine the fate of this terminally differentiated effector cell subset.







Similar content being viewed by others
References
Akbar AN, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, Pilling D, Pett S, Grundy JE, Janossy G (1993) The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections: the role of apoptosis in T cell memory. J Exp Med 178:427
Azuma M, Phillips JH, Lanier LL (1993) CD28- T lymphocytes: antigenic and functional properties, J Immunol 150:1147
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Borthwick NJ, Bofill M, Gombert WM, Akbar AN, Medina E, Sagawa K, Lipman MC, Johnson MA, Janossy G (1994) Lymphocyte activation in HIV-1 infection: II. functional defects of CD28- T cells. AIDS 8:431
Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F (1998) CD28 expression in T cell aging and human longevity. Exp Gerontol 33:267
Bryder DB, Ramsfjell V, Dybedal I, Theilgaard-Monch K, Hogerkorp C-M, Adolfsson J, Borge OJ, Jacobson SEW (2001) Self-renewal of multipotent long-term repopulating hematopoietic stem cells is negatively regulated by Fas and tumor necrosis factor receptor activation. J Exp Med 194:941
Bryl E, Vallejo AN, Weyand CM, Goronzy JJ (2001) Down-regulation of CD28 expression by TNF-α. J Immunol 167:3231
Cardi G, Heaney JA, Schned AR, Ernstoff MS (1998) Expression of Fas (APO-1/CD95) in tumor-infiltrating and peripheral blood lymphocytes in patients with renal cell carcinoma. Cancer Res 58:2078
Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B (1997) Costimulatory signals through B7.1/CD28 prevent T cell apoptosis during target cell lysis. J Immunol 159:3808
Dworacki G, Meidenbauer N, Kuss I, Hoffman TH, Gooding W, Lotze M, Whiteside TL (2001) Decreased ζ expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res 7:947S
Eck SC, Turka LA (1999) Generation of protective immunity against an immunogenic carcinoma requires CD40/CD40L and B7/CD28 interactions but not CD4(+) T cells. Can Immunol Immunother 48:336
Gastman BR, Atarashi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. (1999) Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res 59:5356–5364
Hamann D, Baars PA, Rep MHG, Hooibrink B, Kerkhofgarde SR, Klein MR, van Lier AW (1997) Phenotypic and functional separation of memory and effector human CD8+ cells. J Exp Med 186:1407
Heo DS, Snyderman CH, Gollin SM, Pan S, Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB, Whiteside TL (1989) Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res 49:5167
Hoffmann TK, Dworacki G, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL (2002) Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res 8:2553
Kirchhoff S, Muller WW, Li-Weber M, Krammer PH (2000) Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol 30:2765
Lang S, Atarashi Y, Nishioka Y, Stanson J, Meidenbauer N, Whiteside TL (2000) B7.1 on human carcinomas: costimulation of T cells and enhanced tumor-induced T-cell death. Cellular Immunol 201:132
Lezzi G, Karjalainen K, Lanzavecchia A (1998) The duration of antigenic stimulation determines the fate of naïve and effector cells. Immunity 8:89
McDonagh M, Bell EB (1995) The survival and turnover of mature and immature CD8 T cells. Immunol 84:514
Mugnaini EN, Egeland T, Spurkland A, Brinchmann JE (1999) The T cell repertoire of CD8+CD28- T lymphocytes is dominated by expanded clones that persist over time. Clin Exp Immunol 117:298
Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Lienard D, Lejeune F, Fleishhauer K, Cerundolo V, Cerottine J-C, Romero P (1999) High frequencies of naïve Melan-A/MART-1-specifec CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med 190:705
Prince HE, York J, Jensen ER (1992) Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cellular Immunol 145:254
Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL (1998) Lymphocyte apoptosis induced by Fas Ligand-expressing ovarian carcinoma cells. J Clin Invest 101:2579
Raitakari M, Brown RD, Sze D, Yuen E, Barrow L, Nelson M, Pope B, Esdale W, Gibson J, Joshua DE (2000) T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Haematol 110:203
Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL (2002) Signaling abnormalities, apoptosis and reduced proliferation of circulating and tumor0infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res 8:3137
Saito T, Kuss I, Dworacki G, Gooding W, Johnson JT, Whiteside TL (1999) Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin Cancer Res 5:1263
Saito T, Dworacki G, Gooding W, Lotze M, Whiteside TL (2000) Spontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanoma. Clin Cancer Res 6:1351
Seliger B, Cabrera T, Garrido F, Ferrone S (2002) HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol 12:3
Thor Straten P, Becker JC, Guldberg P, Zeuthen J. (1999) In situ T cells in melanoma. Cancer Immunol Immunother 34:386–395
Trimble LA, Shankar P, Patterson M, Daily JP, Lieberman J (2000) Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-regulated CD3ζ and CD28, key signaling molecules for T-cell activation. J Virol 74:7320
Uzzo RG, Rayman P, Kolenko V, Clark PE, Bloom T, Ward AM, Molto L, Tannenbaum C, Worford LJ, Bukowski R, Tubbs R, His ED, Bander NH, Novick AC, Finke JH (1999) Mechanisms of apoptosis in T cells from patients with renal cell carcinoma. Clin Cancer Res 5:1219
Van den Hove LE, Van Gool SW, Vandenberghe P, Boogaerts MA, Ceuppens JL (1998) CD57+/CD28- T ells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia 12:1573
Verhoven B, Schlegel RA, Williamson P (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182:1597
Walker LS, McLeod JD, Boulougouris G, Patel YI, Hall ND, Sansom DM (1998) Down-regulation of CD28 via Fas (CD95): influence of CD28 on T-cell apoptosis. Immunol 94:41
Walsh PT, O'Connor R (2000) The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol 30:1010
Whiteside TL, Bryant J, Day R, Herberman RB (1990) Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal 4:102
Whiteside TL, Rabinowich H. (1998) The role of Fas/FasL in immunosuppression induced by human tumors. Cancer Immunol and Immunother 46:175–184
Whiteside TL (2002) Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol 12:43
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by NIH grants: PO-1 DE 12321 and RO-1 CA 82016 to Theresa L. Whiteside.
Rights and permissions
About this article
Cite this article
Tsukishiro, T., Donnenberg, A.D. & Whiteside, T.L. Rapid turnover of the CD8+CD28- T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother 52, 599–607 (2003). https://doi.org/10.1007/s00262-003-0395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0395-6