Abstract
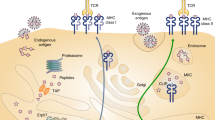
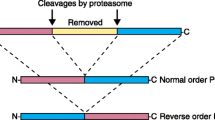
The development of peptide-based vaccines that are useful in the therapeutic treatment of melanoma and other cancers ultimately requires the identification of a sufficient number of antigenic peptides so that most individuals, regardless of their major histocompatibility complex (MHC)–encoded class I molecule phenotype, can develop a cytotoxic T lymphocyte (CTL) response against one or more peptide components of the vaccine. While it is relatively easy to identify antigenic peptides that are presented by the most prevalent MHC class I molecules in the population, it is problematic to identify antigenic peptides that are presented by MHC class I molecules that have less frequent expression in the population. One manner in which this problem can be overcome is by taking advantage of known MHC class I supertypes, which are groupings of MHC class I molecules that bind peptides sharing a common motif. We have developed a mass spectrometric approach which can be used to determine if an antigenic peptide is naturally processed and presented by any given MHC class I molecule. This approach has been applied to the A3 supertype, and the results demonstrate that some, but not all, A3 supertype family–associated peptides can associate with all A3 supertype family members. The approach also demonstrates the shared nature of several newly identified peptide antigens. The use of this technology negates the need to test peptides for their ability to stimulate CTL responses in those cases where the peptide is not naturally processed and bound to the target MHC class I molecule of interest, thus allowing resources to be focused on the most promising vaccine candidates.




Similar content being viewed by others
References
Berger AE, Davis JE, Cresswell P (1982) Monoclonal antibody to HLA-A3. Hybridoma 1:87
Bertoni R, Sidney J, Fowler P, Chesnut RW, Chisari FV, Sette A (1997) Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J Clin Invest 100:503
Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T (1993) The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 178:489
Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ (1997) A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 94:1914
Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Mattei S, De Plaen E, Lurquin C, Szikora JP et al (1994) A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med 180:35
Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL Jr (1994) Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science 264:716
Crotzer VL, Christian RE, Brooks JM, Shabanowitz J, Settlage RE, Marto JA, White FM, Rickinson AB, Hunt DF, Engelhard VH (2000) Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J Immunol 164:6120
Doolan DL, Hoffman SL, Southwood S, Wentworth PA, Sidney J, Chesnut RW, Keogh E, Appella E, Nutman TB, Lal AA, Gordon DM, Oloo A, Sette A (1997) Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7:97
Ellis SA, Taylor C, McMichael A (1982) Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol 5:49
Engelhard VH (1994) Structure of peptides associated with class I and class II MHC molecules. Annu Rev Immunol 12:181
Engelhard VH (1994) Structure of peptides associated with MHC class I molecules. Curr Opin Immunol 6:13
Hendrickson RC, Skipper JC, Shabanowitz J, Slingluff CL Jr (1996) Use of tandem mass spectrometry for MHC ligand analysis. In: Lefkovits I (ed) Immunology methods manual, vol 2. Academic, New York, p 605
Hogan KT, Eisinger DP, Cupp SB III, Lekstrom KJ, Deacon DD, Shabanowitz J, Hunt DF, Engelhard VH, Slingluff CL Jr, Ross MM (1998) The peptide recognized by HLA-A68.2-restricted, squamous cell carcinoma of the lung-specific cytotoxic T lymphocytes is derived from a mutated elongation factor 2 gene. Cancer Res 58:5144
Hogan KT, Coppola MA, Gatlin CL, Thompson LW, Shabanowitz J, Hunt DF, Engelhard VH, Slingluff CL, Ross MM (2003) Identification of a shared epitope recognized by melanoma-specific, HLA-A3-restricted cytotoxic T lymphocytes. Immunol Lett 90:131
Hogan KT, Coppola MA, Gatlin CL, Thompson LW, Shabanowitz J, Hunt DF, Engelhard VH, Ross MM, Slingluff CL (2004) Identification of novel and widely expressed cancer/testis gene isoforms that elicit spontaneous cytotoxic T lymphocyte reactivity to melanoma. Cancer Res 64:1157
Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA (1994) Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A 91:3515
Kawakami Y, Robbins PF, Wang X, Tupesis JP, Parkhurst MR, Kang X, Sakaguchi K, Appella E, Rosenberg SA (1998) Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol 161:6985
Kawashima I, Hudson SJ, Tsai V, Southwood S, Takesako K, Appella E, Sette A, Celis E (1998) The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum Immunol 59:1
Kawashima I, Tsai V, Southwood S, Takesako K, Celis E, Sette A (1998) Identification of gp100-derived, melanoma-specific cytotoxic T-lymphocyte epitopes restricted by HLA-A3 supertype molecules by primary in vitro immunization with peptide-pulsed dendritic cells. Int J Cancer 78:518
Kawashima I, Tsai V, Southwood S, Takesako K, Sette A, Celis E (1999) Identification of HLA-A3-restricted cytotoxic T lymphocyte epitopes from carcinoembryonic antigen and HER-2/neu by primary in vitro immunization with peptide-pulsed dendritic cells. Cancer Res 59:431
Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A (2001) Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A*0201-binding affinity. J Immunol 167:787
Lapatsanis L, Milias G, Froussios K, Kolovos M (1983) Synthesis of N-2,2,2-(trichloroethoxycarbonyl)-L-amino acids and N-(9-Fluorenylmethoxycarbonyl)-L-amino acids involving succinimidoxy anion as a leading group in amino acid protection. Synthesis 8:671
Martin SE, Shabanowitz J, Hunt DF, Marto JA (2000) Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI fourier transform ion cyclotron resonance mass spectrometry. Anal Chem 72:4266
Parham P, Brodsky FM (1981) Partial purification and some properties of BB7.2: a cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol 3:277
Parham P, Barnstable CJ, Bodmer WF (1979) Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol 123:342
Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y (1996) Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol 157:2539
Radka SF, Kostyu DD, Amos DB (1982) A monoclonal antibody directed against the HLA-Bw6 epitope. J Immunol 128:2804
Rebai N, Malissen B (1983) Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens 22:107
Renkvist N, Castelli C, Robbins PF, Parmiani G (2001) A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother 50:3
Russo C, Ng AK, Pellegrino MA, Ferrone S (1983) The monoclonal antibody CR11-351 discriminates HLA-A2 variants identified by T cells. Immunogenetics 18:23
Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M (1995) Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A 92:11810
Sette A, Sidney J (1998) HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Immunol 10:478
Sette A, Sidney J (1999) Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201
Sette A, Newman M, Livingston B, McKinney D, Sidney J, Ishioka G, Tangri S, Alexander J, Fikes J, Chesnut R (2002) Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens 59:443
Shastri N, Schwab S, Serwold T (2002) Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules. Annu Rev Immunol 20:463
Sidney J, Grey HM, Kubo RT, Sette A (1996) Practical, biochemical and evolutionary implications of the discovery of HLA class I supermotifs. Immunol Today 17:261
Skipper JC, Kittlesen DJ, Hendrickson RC, Deacon DD, Harthun NL, Wagner SN, Hunt DF, Engelhard VH, Slingluff CL Jr (1996) Shared epitopes for HLA-A3-restricted melanoma-reactive human CTL include a naturally processed epitope from Pmel-17/gp100. J Immunol 157:5027
Slingluff CL Jr, Colella TA, Thompson L, Graham DD, Skipper JC, Caldwell J, Brinckerhoff L, Kittlesen DJ, Deacon DH, Oei C, Harthun NL, Huczko EL, Hunt DF, Darrow TL, Engelhard VH (2000) Melanomas with concordant loss of multiple melanocytic differentiation proteins: immune escape that may be overcome by targeting unique or undefined antigens. Cancer Immunol Immunother 48:661
Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN (1996) Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565
Threlkeld SC, Wentworth PA, Kalams SA, Wilkes BM, Ruhl DJ, Keogh E, Sidney J, Southwood S, Walker BD, Sette A (1997) Degenerate and promiscuous recognition by CTL of peptides presented by the MHC class I A3-like superfamily: implications for vaccine development. J Immunol 159:1648
Tsai V, Southwood S, Sidney J, Sakaguchi K, Kawakami Y, Appella E, Sette A, Celis E (1997) Identification of subdominant CTL epitopes of the GP100 melanoma-associated tumor antigen by primary in vitro immunization with peptide-pulsed dendritic cells. J Immunol 158:1796
Tureci O, Sahin U, Schobert I, Koslowski M, Scmitt H, Schild HJ, Stenner F, Seitz G, Rammensee HG, Pfreundschuh M (1996) The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res 56:4766
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254:1643
Wang RF, Rosenberg SA (1999) Human tumor antigens for cancer vaccine development. Immunol Rev 170:85
Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA (1996) Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med 184:2207
Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA (1998) A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol 161:3598
Acknowledgements
The authors would like to thank Mitsu Fink, Julie Fitzgerald, and Chantel Tracy for their assistance with the cell culture and isolation of the peptides bound to the A3 supertype molecules. This work was supported by RO1CA90815 (KTH), K08CA91995 (KUC), RO1AI33993 (DFH), and RO1CA57653 (CLS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hogan, K.T., Sutton, J.N., Chu, K.U. et al. Use of selected reaction monitoring mass spectrometry for the detection of specific MHC class I peptide antigens on A3 supertype family members. Cancer Immunol Immunother 54, 359–371 (2005). https://doi.org/10.1007/s00262-004-0592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0592-y