Abstract
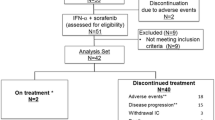
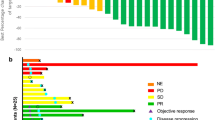
Twenty-two patients with metastatic renal cell carcinoma and removal of the primary tumor were treated with subcutaneous pegylated interferon alfa-2b (PEG-Intron) to evaluate toxicity and efficacy. Start dose was 3.0 μg/kg/week, escalated to 6.0 μg/kg/week. After 2 months, therapy was extended in case of response or stable disease (SD) until progressive disease (PD) or relapse for a maximum of 2 years. National Cancer Institute common toxicity criteria (NCI-CTC) were monitored every 2–4 weeks. After 2 months, nine patients did not continue (8 PD, 1 SD with grade 4 CTC) and 13 extended treatment [three partial response (PR), 10 SD], of these, 11 progressed. One patient with PR developed a durable complete response later. Overall response rate was 13.6% (3/22). Median overall survival is 13 months (range 3–35 months). Dosage was escalated to 6 μg/kg/week in three patients . NCI-CTC grade 2 and 3 required dose attenuation in 12 patients during escalation, and reduction in 10 during the trial. Three patients discontinued because of grade 4 CTC (two fatigue, one hyperglycemia). Fatigue was the major dose-limiting toxicity. These results suggest an efficacy and toxicity of PEG-Intron comparable to standard interferon alfa-2b in patients with mRCC and removal of the primary tumor.
Similar content being viewed by others
References
Bukowski R, Ernstoff MS, Gore ME, Nemunaitis JJ, Amato RJ, Gupta SK, Tendler CL (2002) Pegylated interferon alfa-2b treatment for patients with solid tumors: a phase-I/II study. J Clin Oncol 20:3841–3949
Bukowski R, Tendler CL, Cutler D, Rose E, Laughlin M, Statkevich P (2002) Treating cancer with PEG Intron: pharmakokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer 95:389–396
Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JRj, Munshi N, Crawford ED (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell carcinoma. N Engl J Med 345:1655–1659
Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S (2000) Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther 68:556–567
Kantarjian HM, O’Brien S, Anderlini P, Talpaz M (1996) Treatment of myelogenous leukemia: current status and investigational options. Blood 87:3069–3081
Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M, Blum RH (2000) High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 18:2444–2458
Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, Schiff ER, Goodman ZD, Laughlin M, Yao R, Albrecht JK, Hepatitis Interventional Therapy Group (2001) A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 34:395–403
Medical Research Council Renal Cancer Collaborators (1999) Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet 353:14–17
Mejean A, Oudard S, Thiounn N (2003) Prognostic factors of renal cell carcinoma. J Urol 169:821–827
Mickisch GH, For the European Society of Oncologic Urology (2003) Rational selection of a control arm for randomised trials in metastatic renal cell carcinoma. Eur Urol 43:670–679
Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R (2001) Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 358:966–970
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289–296
Motzer RJ, Rakhit A, Ginsberg M, Rittweger K, Vuky J, Yu R, Fettner S, Hooftman L (2001) Phase I trial of 40-kd branched pegylated interferon alfa-2a for patients with advanced renal cell carcinoma. J Clin Oncol 19:1312–1319
Motzer RJ, Rakhit A, Thompson J, Gurney H, Selby P, Figlin RA, Negrier S, Ernst S, Siebels M, Ginsberg M, Rittweger K, Hooftman L (2002) Phase II trial of branched peginterferon-alfa 2a (40 kDa) for patients with advanced renal cell carcinoma. Ann Oncol 13:1799–1805
Negrier S, Escudier B, Gomez F, Douillard JY, Ravaud A, Chevreau C, Buclon M, Perol D, Lasset C (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais dÍmmunotherapie. Ann Oncol 13:1460–1468
Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela M, Juusela H, Rintala E, Hietanen P, Kellokumpu-Lehtinen PL (1999) Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 19:2859–2867
Simon R (1989) Optimal two-stage designs for phase-II clinical trials. Control Clin Trials 10:1–10
Talpaz M, O’Brien S, Rose E, Gupta S, Shan J, Cortes J, Giles FJ, Faderl S, Kantarjian HM (2001) Phase 1 study of polyethylene glycol formulation of interferon alpha-2b (Schering 54031) in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 98:1708–1713
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
van der Poel HG, Roukema JA, Horenblas S, van Geel AN, Debruyne FM (1999) Metastasectomy in renal cell carcinoma: a multicenter retrospective analysis. Eur Urol 35:197–203
Author information
Authors and Affiliations
Corresponding author
Additional information
Author disclosure declaration: None of the authors has a relationship with pharmaceutical companies, biomedical device manufacturers or other corporations whose products or services are related to the subject matter of the submission, nor do the authors have financial interests such as investments, licensing, or other commercial interest in any drugs, goods, or services in connection with the matter under consideration.
Rights and permissions
About this article
Cite this article
Bex, A., Mallo, H., Kerst, M. et al. A phase-II study of pegylated interferon alfa-2b for patients with metastatic renal cell carcinoma and removal of the primary tumor. Cancer Immunol Immunother 54, 713–719 (2005). https://doi.org/10.1007/s00262-004-0630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0630-9