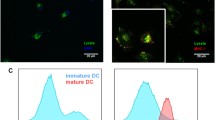
Abstract
The prognosis of malignant gliomas remains dismal and alternative therapeutic strategies are required. Immunotherapy with dendritic cells (DCs) pulsed with tumour antigens emerges as a promising approach. Many parameters influence the efficacy of DC-based vaccines and need to be optimised in preclinical models. The present study compares different vaccine schedules using DCs loaded with tumour cell lysate (DC-Lysate) for increasing long-term survival in the GL26 orthotopic murine glioma model, focusing on the number of injections and an optimal way to recall antitumour immune response. Double vaccination with DC-Lysate strongly prolonged median survival compared to unvaccinated animals (mean survival 87.5 daysvs. 25 days; p < 0.0001). In vitro data showed specific cytotoxic activity against GL26. However, late tumour relapses frequently occurred after 3 months and only 20% of mice were finally cured at 7 months. While one, two or three DC injections gave identical survival, a boost using only tumour lysate after initial DC-Lysate priming dramatically improved long-term survival in vaccinated mice, compared to the double DC-Lysate group, with 67.5% of animals cured at 7 months (p < 0.0001). In vitro data showed better specific CTL response and also the induction of specific anti-GL26 antibodies in the DC-Lysate/Lysate group, which mediated Complement Dependent Cytotoxicity. These experimental data may be of importance for the design of clinical trials that currently use multiple DC injections.







Similar content being viewed by others
References
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359:1011–1018
Parney IF, Hao C, Petruk KC (2000) Glioma immunology and immunotherapy. Neurosurgery 46:778–791
Walker PR, Calzascia T, Dietrich PY (2002) All in the head: obstacles for immune rejection of brain tumours. Immunology 107:28–38
Perrin G, Schnuriger V, Quiquerez AL, Saas P, Pannetier C, de Tribolet N, Tiercy JM, Aubry JP, Dietrich PY, Walker PR (1999) Astrocytoma infiltrating lymphocytes include major T cell clonal expansions confined to the CD8 subset. Int Immunol 11:1337–1350
Ridley A, Cavanagh JB (1971) Lymphocytic infiltration in gliomas: evidence of possible host resistance. Brain 94:117–124
Yoshida J, Kajita Y, Wakabayashi T, Sugita K (1994) Long-term follow-up results of 175 patients with malignant glioma: importance of radical tumour resection and postoperative adjuvant therapy with interferon, ACNU and radiation. Acta Neurochir (Wien) 127:55–59
Yung WK, Prados M, Levin VA, Fetell MR, Bennett J, Mahaley MS, Salcman M, Etcubanas E (1991) Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol 9:1945–1949
Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, Pierz DM, Chen DK, Budzilovich GN, Ransohoff J (1995) Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 76:840–852
Merchant RE, Merchant LH, Cook SH, McVicar DW, Young HF (1988) Intralesional infusion of lymphokine-activated killer (LAK) cells and recombinant interleukin–2 (rIL-2) for the treatment of patients with malignant brain tumor. Neurosurgery 23:725–732
Bloom HJ, Peckham MJ, Richardson AE, Alexander PA, Payne PM (1973) Glioblastoma multiforme: a controlled trial to assess the value of specific active immunotherapy in patients treated by radical surgery and radiotherapy. Br J Cancer 27:253–267
Trouillas P (1973) Immunology and immunotherapy of cerebral tumors. Current status. Rev Neurol (Paris) 128:23–38
Weller M, Fontana A (1995) The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev 21:128–151
Schuler G, Steinman R (1997) Dendritic cells as adjuvants for immune-mediated resistance to tumors. Exp Med 186(8):1183–1187
Young JW, Inaba K (1996) Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med 183:7–11
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D (1998) Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4:328–332
Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Seipp CA, Einhorn JH, Roberts B, White DE (1998) Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst 90:1894–1900
Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert RH (2000) Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med 6:332–336
Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T (2001) Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother 24:106–113
Aoki H, Mizuno M, Natsume A, Tsugawa T, Tsujimura K, Takahashi T, Yoshida J (2001) Dendritic cells pulsed with tumor extract-cationic liposome complex increase the induction of cytotoxic T lymphocytes in mouse brain tumor. Cancer Immunol Immunother 50:463–468
Heimberger AB, Crotty LE, Archer GE, McLendon RE, Friedman A, Dranoff G, Bigner DD, Sampson JH (2000) Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol 103:16–25
Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M (2002) A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res 22:613–621
Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 50:337–344
Yamanaka R, Yajima N, Tsuchiya N, Honma J, Tanaka R, Ramsey J, Blaese M, Xanthopoulos KG (2002) Administration of interleukin-12 and −18 enhancing the antitumor immunity of genetically modified dendritic cells that had been pulsed with Semliki forest virus-mediated tumor complementary DNA. J Neurosurg 97:1184–1190
Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842–847
Cerundolo V, Hermans IF, Salio M (2004) Dendritic cells: a journey from laboratory to clinic. Nat Immunol 5:7–10
Fong L, Engleman EG (2000) Dendritic cells in cancer immunotherapy. Annu Rev Immunol 18:245–273
Ausman JI, Shapiro WR, Rall DP (1970) Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res 30:2394–2400
Prins RM, Odesa SK, Liau LM (2003) Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res 63:8487–8491
Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176:1693–1702
Tutt AL, French RR, Illidge TM, Honeychurch J, McBride HM, Penfold CA, Fearon DT, Parkhouse RM, Klaus GG, Glennie MJ (1998) Monoclonal antibody therapy of B cell lymphoma: signaling activity on tumor cells appears more important than recruitment of effectors. J Immunol 161:3176–3185
Fischer K, Mackensen A (2003) The flow cytometric PKH-26 assay for the determination of T-cell mediated cytotoxic activity. Methods 31:135–142
Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, Grabbe S (1999) Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol 162:168–175
O’Neill DW, Adams S, Bhardwaj N (2004) Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 104:2235–2246
Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G (2001) Dendritic cells as vectors for therapy. Cell 106:271–274
Shimizu K, Thomas EK, Giedlin M, Mule JJ (2001) Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res 61:2618–2624
Kurokawa T, Oelke M, Mackensen A (2001) Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer 91:749–756
Glennie MJ, Johnson PW (2000) Clinical trials of antibody therapy. Immunol Today 21:403–410
Gelderman KA, Tomlinson S, Ross GD, Gorter A (2004) Complement function in mAb-mediated cancer immunotherapy. Trends Immunol 25:158–164
Sornasse T, Flamand V, De Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Leo O, Moser M (1992) Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med 175:15–21
Chakraborty NG, Li L, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B (1999) Emergence of regulatory CD4+ T cell response to repetitive stimulation with antigen-presenting cells in vitro: implications in designing antigen-presenting cell-based tumor vaccines. J Immunol 162:5576–5583
Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N (2000) Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest 105:R9–R14
Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F (2000) CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol 164:3095–3101
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J (2001) Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 61:6451–6458
Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumour lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979
Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179
Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T (2004) Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother 27:452–459
Figdor CG, De Vries IJ, Lesterhuis WJ, Melief CJ (2004) Dendritic cell immunotherapy: mapping the way. Nat Med 10:475–480
Acknowledgements
We thank Drs Christine Menetrier-Caux, Christophe Caux, and Nathalie Vermare-Bendriss for helpful comments on the manuscript and Dominique Reynaud for technical assistance. This study was supported, in part, by grants from the French national league against cancer, the departmental leagues against cancer of Ardèche and Drôme, the French Association for Research on Cancer (ARC) and the breast cancer research foundation (BCRF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jouanneau, E., Poujol, D., Gulia, S. et al. Dendritic cells are essential for priming but inefficient for boosting antitumour immune response in an orthotopic murine glioma model. Cancer Immunol Immunother 55, 254–267 (2006). https://doi.org/10.1007/s00262-005-0040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0040-7