Abstract
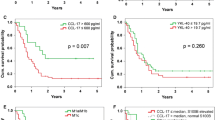
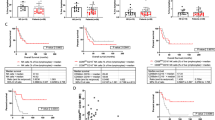
It was previously shown that CEACAM1 on melanoma cells strongly predicts poor outcome. Here, we show a statistically significant increase of serum CEACAM1 in 64 active melanoma patients, as compared to 48 patients with no evidence of disease and 37 healthy donors. Among active patients, higher serum CEACAM1 correlated with LDH values and with decreased survival. Multivariate analysis with neutralization of LDH showed that increased serum CEACAM1 carries a hazard ratio of 2.40. In vitro, soluble CEACAM1 was derived from CEACAM1(+), but neither from CEACAM1(−) melanoma cells nor from CEACAM1(+) lymphocytes, and directly correlated with the number of CEACAM1(+) melanoma cells. Production of soluble CEACAM1 depended on intact de novo protein synthesis and secretion machineries, but not on metalloproteinase function. An unusually high percentage of CEACAM1(+) circulating NK and T lymphocytes was demonstrated in melanoma patients. CEACAM1 inhibited killing activity in functional assays. CEACAM1 expression could not be induced on lymphocytes by serum from patients with high CEACAM1 expression. Further, expression of other NK receptors was impaired, which collectively indicate on a general abnormality. In conclusion, the systemic dysregulation of CEACAM1 in melanoma patients further denotes the role of CEACAM1 in melanoma and may provide a basis for new tumor monitoring and prognostic platforms.








Similar content being viewed by others
References
National Cancer Institute. Surveillance epidemiology and end results. http://seer.cancer.gov/csr/1975_2004/results_merged/sect_16_melanoma.pdf. Last accessed at 28 January 2009
National Cancer Institute. Surveillance epidemiology and end results. http://seer.cancer.gov/publications/aya/5_melanoma.pdf. Last accessed at 28 January 2009
Herlyn M (1990) Human melanoma: development and progression. Cancer Metastasis Rev 9:101–112
Breslow A (1970) Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 172:902–908
Soong S-J, Shaw HM, Balch CM et al (1992) Predicting survival and recurrence in localized melanoma: a multivariate approach. World J Surg 16:191–195
Schuchter L, Schultz DJ, Synnestvedt M et al (1996) A prognostic model for predicting 10-year survival in patients with primary melanoma. Ann Intern Med 125:369–375
Christianson DF, Anderson CM (2003) Close monitoring and lifetime follow-up is optimal for patients with a history of melanoma. Semin Oncol 30:369–374
Garbe C, Paul A, Kohler-Späth H et al (2003) Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J Clin Oncol 21:520–529
Deichmann M, Benner A, Bock M et al (1999) S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol 17:1891–1896
Deichmann M, Kahle B, Moser K et al (2004) Diagnosing melanoma patients entering American Joint Committee on Cancer stage IV, C-reactive protein in serum is superior to lactate dehydrogenase. Br J Cancer 91:699–702
Beauchemin N, Draber P, Dveksler G et al (1999) Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 252:243–249
Vogelzang NJ, Lange PH, Goldman A (1982) Acute changes of α-fetoprotein and human chorionic gonadotropin during induction chemotherapy of germ cell tumors. Cancer Res 42:4855–4861
Locker GY, Hamilton S, Harris J et al (2006) ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 24:5313–5327
Hammarström S (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9:67–81
Thies A, Moll I, Berger J et al (2002) CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol 20:2530–2536
Laack E, Nikbakht H, Peters A et al (2002) Expression of CEACAM1 in adenocarcinoma of the lung: a factor of independent prognostic significance. J Clin Oncol 20:4279–4284
Sienel W, Dango S, Woelfle U et al (2003) Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res 9:2260–2266
Ebrahimnejad A, Streichert T, Nollau P et al (2004) CEACAM1 enhances invasion and migration of melanocytic and melanoma cells. Am J Pathol 165:1781–1787
Markel G, Wolf D, Hanna J et al (2002) Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest 110:943–953
Markel G, Lieberman N, Katz G et al (2002) CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol 168:2803–2810
Markel G, Seidman R, Stern N et al (2006) Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 interactions. J Immunol 177:6062–6071
Markel G, Seidman R, Cohen Y et al. (2008) Dynamic expression of protective CEACAM1 on melanoma cells during specific immune attack. Immunology [Epub ahead of print]
Dráberová L, Cerná H, Brodská H et al (2000) Soluble isoforms of CEACAM1 containing the A2 domain: increased serum levels in patients with obstructive jaundice and differences in 3-fucosyl-N-acetyl-lactosamine moiety. Immunology 101:279–287
Kondo Y, Hinoda Y, Akashi H et al (2001) Measurement of circulating biliary glycoprotein (CD66a) in liver diseases. J Gastroenterol 36:470–475
Svenberg T, Wahren B, Hammarström S (1979) Elevated serum levels of a biliary glycoprotein (BGP I) in patients with liver or biliary tract disease. Clin Exp Immunol 36:317–325
Simeone DM, Ji B, Banerjee M et al (2007) CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas 34:436–443
Moller MJ, Kammerer R, Grunert F et al (1996) Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer 65:740–745
Kammerer R, Hahn S, Singer BB et al (1998) Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol 28:3664–3674
Albarran-Somoza B, Franco-Topete R, Delgado-Rizo V et al (2006) CEACAM1 in cervical cancer and precursor lesions: association with human papillomavirus infection. J Histochem Cytochem 54:1393–1399
Markel G, Gruda R, Achdout H et al (2004) The critical role of residues 43R and 44Q of carcinoembryonic antigen cell adhesion molecules-1 in the protection from killing by human NK cells. J Immunol 173:3732–3739
Takino T, Saeki H, Miyamori H et al (2007) Inhibition of membrane-type 1 matrix metalloproteinase at cell–matrix adhesions. Cancer Res 67:11621–11629
Markel G, Achdout H, Katz G et al (2004) Biological function of the soluble CEACAM1 protein and implications in TAP2-deficient patients. Eur J Immunol 34:2138–2148
Groh V, Wu J, Yee C et al (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734–738
Vetter CS, Groh V, thor Straten P et al (2002) Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol 118:600–605
Izzi L, Turbide C, Houde C et al (1999) cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene 18:5563–5572
Horst AK, Ito WD, Dabelstein J et al (2006) Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest 116:1596–1605
Wikström K, Kjellström G, Obrink B (1996) Homophilic intercellular adhesion mediated by C-CAM is due to a domain 1-domain 1 reciprocal binding. Exp Cell Res 227:360–366
Thies A, Mauer S, Fodstad O et al (2007) Clinically proven markers of metastasis predict metastatic spread of human melanoma cells engrafted in scid mice. Br J Cancer 96:609–616
Gray-Owen SD, Blumberg RS (2006) CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 6:433–446
Barnett TR, Drake L, Pickle WII (1993) Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins. Mol Cell Biol 13:1273–1282
Budt M, Michely B, Müller MM (2002) Secreted CEACAM1 splice variants in rat cell lines and in vivo in rat serum. Biochem Biophys Res Commun 292:749–755
Stamey TA, Yang N, Hay AR et al (1987) Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909–916
Azuz-Lieberman N, Markel G, Mizrahi S et al (2005) Involvement Natural Killer cells and CEACAM1 in Ankylosing Spondylitis. Int Immunol 17:837–845
Markel G, Mussaffi H, Ling KL et al (2004) The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood 10:31770–31778
Takahashi H, Okai Y, Paxton RJ et al (1993) Differential regulation of carcinoembryonic antigen and biliary glycoprotein by gamma-interferon. Cancer Res 53:1612–1619
Konjević G, Mirjacić Martinović K, Vuletić A et al (2007) Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis 24:1–11
Acknowledgments
Many special thanks to Haya and Nehemia Lemelbaum for the enormous support allowing the authors to conduct these studies. The authors would also like to thank the volunteers who donated blood samples and Mr. Amit Markel for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Markel and R. Ortenberg have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Markel, G., Ortenberg, R., Seidman, R. et al. Systemic dysregulation of CEACAM1 in melanoma patients. Cancer Immunol Immunother 59, 215–230 (2010). https://doi.org/10.1007/s00262-009-0740-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0740-5