Abstract
The possibility of controlling the harmful intra-articular influence of elevated interleukin (IL)-1β synovial fluid concentration after anterior cruciate ligament (ACL) surgery could be useful. We investigated the correlation between serum and synovial fluid IL-1β levels following ACL reconstruction. We measured IL-1β concentration periodically in three synovial fluid and four serum samples in each of 20 patients receiving either autologous conditioned serum (ACS) containing endogenous anti-inflammatory cytokines including IL-1Ra and several growth factors (group A) or placebo (group B). A decrease in IL-1β synovial fluid concentration appeared to be more pronounced in absolute terms in group A. In eight patients serum IL-1β was detected on the 6th postoperative day. In four of them whose synovial fluid levels were over 10 pg/ml on the 6th postoperative day, serum IL-1β was detected on the 10th postoperative day. The results were different in group B. Correlation between serum and synovial fluid IL-1β appearance persists in patients after ACL surgery and ACS application. This study is an example of ACS influence on the ACL healing process controlling the IL-1β levels on the basis of the serum IL-1β detection.
Résumé
Le contrôle de l’influence intra-articulaire néfaste de la concentration synoviale en l’IL-1β après chirurgie du croisé antérieur. Matériel et méthode: nous avons analysé les corrélations entre le sérum et le liquide synovial avec dosage des taux de l’IL-1β après la reconstruction du croisé antérieur. Nous avons mesuré la concentration de l’IL-1β de façon périodique avec trois prélèvements synoviaux et 4 dosages sériques chez 20 patients recevant un sérum ACS contenant des cytokines anti-inflammatoires incluant IL-1Ra et les différents facteurs de croissance (Groupe A) ou le Placébo (Groupe B). Résultats: la concentration de l’IL-1β dans le liquide synovial diminue de façon importante dans le groupe A. Chez 8 patients l’IL-1β sérique a été détectée au sixième jour post-opératoire. Pour chacun d’entre-eux le taux d’ IL-1β synovial était supérieur à 10 pg/ml, à la sixième heure post-opératoirel’IL-1β sérique a été détecté au 10ème jour post-opératoire. Les résultats sont très différents dans le groupe B. En conclusion, une corrélation persiste entre les taux sériques et les taux synoviaux de l’IL-1β chez les patients après chirurgie du croisé antérieur. Cette étude est un exemple de l’influence de l’ACS dans le processus de cicatrisation en contrôlant les taux de l’IL-1β et en analysant les taux sériques de celui-ci.
Similar content being viewed by others
Introduction
A traumatic anterior cruciate ligament (ACL) rupture is a common lesion of the knee joint. ACL reconstruction is an effective method to eliminate the concomitant anterior tibial subluxation of the knee joint [23].
According to the available literature, there are 10–25% of unsuccessful results after an ACL reconstruction due to different possible causes. The postoperative tibial and femoral tunnel widening visible on radiological films is one of the more recent causes. It results in potential higher knee laxity that could be an early sign of a bad postoperative result. Bone resorption and osteolytic processes in this area are the reasons for tunnel widening [24]. There is growing evidence that interleukin-1β (IL-1β) plays an important role in the pathogenesis of bone tunnel enlargement following ACL reconstruction [7, 13, 16].
IL-1α and IL-1β act on the same cell receptors with high affinity. Most of the IL-1 activity found in the body fluids is IL-1β. Almost all cells can produce IL-1β when they are damaged, but macrophages produce the biggest amount of IL-1β as they are probably the best producers of this cytokine. IL-1β initiates immunoreactions during bone remodelling. This process is based on the initiation of bone matrix synthesis by the activation of osteoclasts and the inhibition of osteoblasts. Cameron et al. have shown that the concentrations of proinflammatory cytokines, such as IL-1β, are elevated in the synovial fluid immediately after an ACL injury and continue to be elevated several weeks thereafter [4, 5, 18]. Because of that, IL-1β is a potential target for therapeutic intervention in several inflammatory and autoimmune stages [16, 17, 21].
IL-1 cytokine receptor antagonists (IL-1Ra) are well-known natural inhibitors of IL-1β and play a role as suppressors of IL-1β. IL-1Ra competes with IL-1β for binding to IL-1 receptor type I (IL-1RI) on target cells. Unlike IL-1β, binding of IL-1Ra to IL-1RI prevents the docking of the IL-1 receptor accessory protein (IL-1R AcP) to form the heterotrimeric complex that is necessary for signal transduction and therefore inhibits IL-1β production and their role in immunoreactions. Recent studies have established that IL-1Ra significantly reduces local joint inflammation and bone erosion in patients suffering from rheumatoid arthritis in the worst stages. Many authors have confirmed that there is a natural balance between IL-1β proinflammatory activity and the IL-1Ra ability to block this activity by occupying IL-1β receptors simultaneously [1, 6–9, 12, 21, 22].
The biological medical product autologous conditioned serum (ACS) containing endogenous anti-inflammatory cytokines including IL-1Ra and several growth factors (insulin-like growth factor-1, platelet-derived growth factor and transforming growth factor-ß1) in the liquid blood phase is based on the principle of inhibiting the biological activity of IL-1β. The main anti-inflammatory cytokine in ACS is IL-1Ra because its concentration in ACS is increased 140-fold greater than in normal serum, while the concentrations of other anti-inflammatory cytokines and growth factors are slightly induced by treatment with glass beads [19]. In our study we used it for intra-articular knee injection in order to reduce the IL-1β influence on the process of ACL healing.
The possibility of controlling IL-1β intra-articular inflammatory and osteoclastic behaviour could be useful. It has been established that the proinflammatory cytokine IL-1β in peripheral blood might be used as a diagnostic marker for aseptic loosening of large joint prostheses [14].
One might assess local tissues for the proinflammatory cytokine IL-1β to control its harmful influence, but diagnostic material would need to be obtained by invasive surgical procedures. Measurement of the IL-1β level in synovial fluid also requires an invasive procedure (sterile knee puncture), which might be inappropriate for routine diagnostic work-up.
Although during the postoperative ACL healing process IL-1β is mostly localised in the area of its activation in synovial fluid of the knee joint, we presumed that a certain amount of this proinflammatory cytokine reaches the systemic circulation via capillary blood flow, thus indicating the process of active bone resorption.
We hypothesised an increased concentration of the proinflammatory cytokine IL-1β in the peripheral circulation of patients following an ACL reconstruction indicating its stronger osteoclastic influence. Thus, determining levels of proinflammatory cytokines in peripheral blood may be a clinically acceptable method for predicting and diagnosing a possibly bad result of ACL surgery.
Our aim was to investigate the presence of IL-1β in the peripheral circulation during the ACL healing process, a possible correlation with its elevated levels in synovial fluid and the difference and variability of IL-1β regarding ACS treatment.
Materials and methods
We performed a prospective, randomised, double-blind, placebo-controlled group study. Two groups, A and B, of ten patients each were treated. Each patient was fully informed about the purpose of the trial, expected benefits, possible risks as well as all the other details pertaining to the study. They signed the informed consent. The approval for this study was obtained from the Ethics Committee, School of Medicine, University of Zagreb, prior to performing the study.
This study used demographic data from the study diaries according to the operative and postoperative protocols. The patients underwent surgical reconstruction of a traumatic rupture of the ACL of a knee joint using an autograft of the patellar ligament. The operations were performed by the same surgeon during a period of six months after the injury. Prior to each operation, the traumatic rupture of the ACL was determined on the basis of a clinical examination and confirmed by explorative and therapeutic arthroscopic surgery in accordance with good surgical practice. Our study included patients between the ages of 18 and 55 with the same preoperative diagnosis of isolated ACL rupture, knee axis deviation up to 5, knee arthrosis up to grade 1 according to the AO and knee chondral lesion up to grade 2 with the same operational protocol. The postoperative protocol was different for groups A and B.
The group A patients were treated with intra-articular application of 2 ml of ACS on the day of the surgery and on postoperative days one, six and ten. ACS production was performed according to the manufacturer’s instructions. The control group B patients were treated with placebo (2 ml of physiological solution) on the day of the surgery and on postoperative days one, six and ten.
The measurements of IL-1β concentration in the peripheral circulation were performed the day of operation and postoperative days one, six and ten. The serum samples were obtained using sterile venous puncture, centrifugation at 4,800 g for 10 min and freezing at −80°. The measurements of the IL-1β intra-articular concentration were performed on postoperative days one, six and ten after the synovial fluid samples were obtained using sterile knee puncture, centrifugation at 4,800 g for ten minutes and freezing at −80°. The IL-1β intra-articular concentration was not measured on the day of operation for ethical reasons.
The measurements of IL-1β in serum and synovial fluid were performed by an independent expert using Human IL-1β /IL-1F2 Quantikine ELISA Kit, (Minneapolis, MN, USA). The lowest possible amount measured was 1 pg/ml. This value was handled as a lower limit of quantification (LOQ) for the purpose of data analysis, i.e. the values below 1 pg/ml were substituted by 0.5 pg/ml (which is LOQ/2). The lowest test standard was 3.9 pg/ml and the highest 250 pg/ml. The results were 0 in the cases where the absorbance was below the value measured in the blank test. Statistical analyses using SAS 8.2 on a Windows XP computer were applied on an exploratory basis, i.e. unpaired t-tests assuming equal and unequal variances for comparison of the treated and the non-treated groups. A paired t-test for comparing days six and ten with day one within each patient group was applied. A strong biased result distribution yielded by descriptive analysis necessitated logarithmic transformation of the results. P values were derived from two-sided tests. Type I error (α = 0.05) was considered significant for all statistical tests.
Results
Synovial fluid concentrations
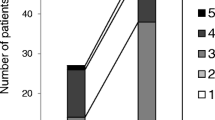
The synovial fluid IL-1β concentration in all of the patients of group A (average age 32) on the first postoperative day was significantly higher than the normal level of 10 ± 4,. pg/ml, ranging from 14.53 to 92.45 (median: 37.67) pg/ml. On the sixth postoperative day the IL-1β concentration was lower than on the first day, ranging from 0.74 to 13.82 (median: 7.82) pg/ml. On the tenth postoperative day the IL-1β concentration decreased rapidly and had the lowest level in all of the patients, ranging from 0.72 to 11.32 (median: 5.67) pg/ml.
The synovial fluid IL-1β concentration in control group B (average age 34) was higher than the normal level on the first postoperative day in nine patients, ranging from 17.88 to 54.53 pg/ml, and in one patient it was normal with a level of 4.37 pg/ml (median: 32.53 pg/ml). On the sixth day after the surgery the IL-1β concentration in all of the patients was lower than on the first day, but not so notable as in group A, ranging from 0 to 24.07 (median: 12.26) pg/ml. On the tenth postoperative day the IL-1β concentration in seven patients was higher, ranging from 1.69 to 77.32 pg/ml, and in three patients was lower, ranging from 2.01 to 19.72 pg/ml, than on the sixth postoperative day (median: 25.05 pg/ml) (Fig. 1).
The summary statistics with regard to the IL-1β synovial fluid concentration showed that the IL-1β concentration tended to decrease by a comparable extent from day one to day ten in all group A patients. That was not equal in seven patients in group B. The variability of concentrations was high, which led to unstable estimates of the mean values. In particular, mean and median values on day one differed significantly in both groups. The variability in group A was found to be larger than in control group B.
The differences between days six to ten and day one were analysed within groups. A decrease in the IL-1β concentrations appeared to be more pronounced in absolute terms in group A when compared to control group B. The variability (expressed as the standard deviation, SD) was considerably higher in group A than in control group B and the values were statistically significantly lower in group A on day ten (P = 0.017).
Serum concentrations
IL-1β concentrations in the peripheral circulation days 0 and 1 were 0.00 pg/ml in all of the serum specimens in both groups.
In eight out of ten group A patients serum IL-1β was detected on the sixth postoperative day, ranging from 0.08 to 1.48 pg/ml (median: 0.53 pg/ml). In four of them whose synovial fluid levels were over 10 pg/ml on the sixth postoperative day, serum IL-1β was detected on the tenth postoperative day, ranging from 0.31 to 3.42 pg/ml (median: 0.45 pg/ml). In three of four patients the IL-1β concentration increased from 1.33 to 3.42 pg/ml, from 0.23 to 0.34 pg/ml and from 0.00 to 0.39 pg/ml, but in one of them the IL-1β concentration decreased from 1.48 to 0.31 pg/ml.
At the same time there was no such correlation in group B, and the IL-1β concentrations in the peripheral circulation in all serum specimens measured were 0.00 pg/ml (Fig. 2).
Discussion
The concentration of IL-1β in the normal human synovial fluid is 10 ± 4.3 pg/ml [4, 5, 10, 18, 25]. Several magnetic resonance imaging studies have shown synovial fluid tracking between the graft-bone tunnel interface [25]. The bone tunnel is subsequently exposed to increased levels of cytokines within the synovial fluid possibly inducing osteolysis [3, 13, 15].
This theory is supported by the finding of Berg et al. The authors investigated interarticular bone tunnel healing in a rabbit model and found that tunnel healing was slower and less complete in the articular part of the tunnel than in the tunnel parts which were farther away from the synovial environment. When allografts were in the joints, elevated IL-1β and tumour necrosis factor (TNF)-α levels were detected, which supports the view that these cytokines were related to bone resorption of the allograft [2]. The result is bone tunnel enlargement and a concomitant anterior tibial subluxation of the knee joint [8, 15, 17, 20].
Autologous conditioned serum or ACS is produced from the patient’s own blood and can be manufactured with ease at the point of care. So far, the ACS production process results in an accumulation of anti-inflammatory cytokines and growth factors in the serum. When injected, biological factors appear to have symptom- and possibly disease-modifying effects that represent an alternative and highly promising approach to treatment of orthopaedic diseases [19]. In our study the main goal of ACS treatment was to modify the IL-1β osteoclastic influence after ACL surgery.
We have tested our hypothesis that elevated synovial fluid IL-1β levels in patients following an ACL reconstruction could be a cause of an increased concentration of IL-1β in the peripheral circulation.
According to our results, the correlation between ACS and placebo treatment, IL-1β levels in the synovial fluid increased immediately after an operative ACL reconstruction in all samples. Our study suggests that the levels of IL-1β concentration in the synovial fluid after an operative ACL reconstruction were higher than normal in both groups and remained as such for approximately ten days. These dynamics were similar to those previously reported. In the patients with intra-articular ACS application (group A), a decrease of IL-1β concentration levels over time showed regularity as the healing effect of ACS. Finally, after ten days, in group A these values were equal to or below the concentration in a normal knee and statistically significantly lower than in group B. In the patients treated with intra-articular placebo application there was no such correlation in the synovial fluid IL-1β concentration levels. It could be considered as an example of positive ACS influence on the ACL post-traumatic healing process after an acute rupture and operative reconstruction. The main factor in ACS is IL-1Ra which antagonises the action of IL-1β in such a way that it competes for binding to the same receptor sites. The result is inhibition of IL-1β production and therefore inhibition of IL-1β-mediated effects [22].
The serum IL-1β detected only in the patients who received ACS could be explained in terms of the role of IL-1Ra, also. Binding of IL-1Ra to IL-1RI prevents binding of IL-1β, activation of IL-1R AcP, signal transduction in the target cell and therefore additional IL-1β production.
A certain amount of IL-1β which cannot bind to the unavailable receptor might reach the systemic circulation via capillary blood flow. In plasma, active proinflammatory cytokines bind to plasma proteins and soluble cytokine receptors, undergoing proteolytic degradation; thus, their half-life is extremely short, i.e. only a few minutes. Upon degradation, inactivated cytokine monomers are found in plasma, which can be detected several days after cessation of their biological activity [14, 22]. Because of that we presumed that IL-1β could reach the peripheral circulation only in the patients who received ACS.
The serum IL-1β levels were considerably lower than our test sensitivity levels. Gonzales et al. determined plasma levels of proinflammatory cytokines in healthy subjects using preanalytical processing of plasma samples, which were identical to those used in our study, and also obtained levels of proinflammatory cytokines lower than the reference values of test sensitivity. They postulated that the reference values set by the manufacturer were based on the mean value in a population sample, thus being of limited value. The individual levels of the tested substance may be detected only if they exceed the mean level of this substance determined in the respective population [11, 14]. Therefore, we considered the plasma levels of IL-1 that were equal to or below the limit of test sensitivity as normal and those exceeding the limit of test sensitivity as increased levels. Thus, we could conclude that a correlation between the IL-1β appearance in the peripheral circulation and synovial fluid persists in patients with an operative ACL reconstruction and ACS application.
Due to the small number of patients involved in our study, it is difficult to make strong conclusions. Our preliminary results should be confirmed in a larger randomised controlled trial with a higher number of subjects. Our study could, however, be considered as an example of ACS influence on the ACL healing process controlling the IL-1β levels on the basis of the serum IL-1β detection.
References
Arend WP (1993) Interleukin-1 receptor antagonist. Adv Immunol 54:167–227
Berg EE, Pollard ME, Kang Q (2001) Interarticular bone tunnel healing. Arthroscopy 17:189–195
Borovecki F, Pecina-Slaus N, Vukicevic S (2007) Biological mechanisms of bone and cartilage remodelling—genomic perspective. Int Orthop 31:799–805
Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH (1997) The natural history of the anterior ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med 25:751–754
Cameron ML, Fu FH, Paessler HH, Schneider M, Evans CH (1994) Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc 2:38–44
Dinarello CA (1994) Blocking interleukin-1 receptors. Int J Clin Lab Res 24:61–79
Dinarello CA (1994) The interleukin-1 family: 10 years of discovery. FASEB J 8:1314–1325
Dinarello CA (2001) The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734
Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, Sheppard M, Krishnan BR, Pelletier JP (1999) In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am J Pathol 154:1159–1169
Frank C, Amiel D, Woo SL, Akeson W (1985) Normal ligament properties and ligament healing. Clin Orthop Relat Res 196:15–25
Gonzalez C, Cava F, Ayllon A, Guevara P, Navajo JA, Gonzalez-Buitrago JM (2001) Biological variation of interleukin-1b, interleukin-8 and tumor necrosis factor-a in serum of healthy individuals. Clin Chem Lab Med 39:836–841
Granowitz EV, Clark BD, Mancilla J, Dinarello CA (1991) Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II intereleukin-1 receptor. J Biol Chem 266:14147–14150
Hoher J, Moller HD, Fu F (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc 6:231–240
Hundrić-Haspl Z, Pecina M, Haspl M, Tomicic M, Jukic I (2006) Plasma cytokines as markers of aseptic prosthesis loosening. Clin Orthop Relat Res 453:299–304
Jacobs JJ, Roebuck KA, Archibeck M et al (2001) Osteolysis: basic science. Clin Orthop 393:71–77
Irie K, Uchiyama E, Iwaso H (2003) Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10:93–96
Manologas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Marks P, Cameron M (2000) Inflammatory cytokine profiles correlate with the degree of chondrosis in the chronic anterior cruciate ligament deficient knee. ACL study meeting, Rhodes, Greece
Meijer H, Reinecke J, Becker C, Tholen G, Wehling P (2003) The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 52:404–407
Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S (2004) Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med 32:1619–1625
Poutsiaka DD, Clark BD, Vannier E, Dinarello CA (1991) Production of interleukin-1 receptor antagonist and interleukin-1 beta by peripheral blood mononuclear cells is differentially regulated. Blood 78:1275–1281
Volk HD, Keysser G, Burmester GR (1998) Cytokines and cytokine receptors. In: Thomas L (ed) Clinical laboratory diagnostics. Use and assessment of clinical laboratory results. TH-Books Verlagsgesellschaft, Frankfurt/Main, pp 764–773
Williams RJ 3rd, Hyman J, Petrigliano F, Rozental T, Wickiewicz TL (2004) Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. J Bone Joint Surg Am 86-A:225–232
Wilson TC, Kantaras A, Atay A, Johnson DL (2004) Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med 32:543–549
Zysk SP, Fraunberger P, Veihelmann A, Dorger M, Kalteis T, Maier M, Pellengahr C, Refior HJ (2004) Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc 12:98–103
Acknowledgement
The source of all funding for the study was obtained by the National Health Insurance.
We thank Vera Srsan Zivanovic (Department of General Surgery and Traumatology, General Hospital Varazdin), Damir Poljak (Department of Transfusion Medicine, General Hospital Varazdin) and Renata Jug (Institute of Immunology, Zagreb) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darabos, N., Hundric-Haspl, Z., Haspl, M. et al. Correlation between synovial fluid and serum IL-1β levels after ACL surgery–preliminary report. International Orthopaedics (SICOT) 33, 413–418 (2009). https://doi.org/10.1007/s00264-008-0649-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-008-0649-1