Abstract
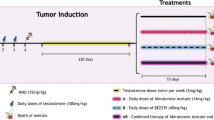
Purpose: Therapeutic efficacy of the novel matrix metalloproteinase (MMP) inhibitor Ro 28-2653 has been shown in various models of different tumor entities. We hypothesized that the inhibitor effect of Ro 28-2653 on the tumor growth could be improved by combination with chemotherapeutic drugs and examined therefore the effect of Ro 28-2653 alone and in combination with etoposide or estramustine in the MatLyLu Dunning R-3327 rat tumor model characteristic for the androgen-independent prostate cancer (PCa). Methods: In vitro effects were estimated measuring the proliferation of MatLyLu cells incubated with the three agents alone or in combination using the XTT test. The in vivo effects of the agents alone or in combination were examined by measuring the tumor weight 18 days after tumor cell injection. Results: The proliferation rate was only inhibited by etoposide while that effect was increased in combination with Ro 28-2653 and estramustine. Ro 28-2653 reduced the tumor weight by 86%. That effect was significantly increased in combination with etoposide to 92%. Conclusions: The inhibitory effect of the MMP inhibitor Ro 28-2653 on the tumor growth in the Dunning PCa model is enhanced by the standard chemotherapeutic drug etoposide. A combined application of both agents could be considered as potential tool to improve the therapy of patients with advanced PCa after failure of hormonal treatment.



Similar content being viewed by others
References
Abramjuk C, Jung K, Krell HW, Juchem R, Peters R, Taymoorian K, Staack A, Stephan C, Schnorr J, Loening SA, Lein M (2005) Matrix metalloproteinase inhibitor Ro 28-2653 in combination with estramustine: tumor-reducing effects on hormone-sensitive prostate cancer in rats. Anticancer Drugs 16:855–861
Andarawewa KL, Boulay A, Masson R, Mathelin C, Stoll I, Tomasetto C, Chenard MP, Gintz M, Bellocq JP, Rio MC (2003) Dual stromelysin-3 function during natural mouse mammary tumor virus-ras tumor progression. Cancer Res 63:5844–5849
Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C (2003) Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 35:252–257
Chang C, Werb Z (2001) The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 11:37–43
Chow KC, King CK, Ross WE (1988) Abrogation of etoposide-mediated cytotoxicity by cycloheximide. Biochem Pharmacol 37:1117–1122
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295:2387–2392
Dahllof B, Billstrom A, Cabral F, Hartley-Asp B (1993) Estramustine depolymerizes microtubules by binding to tubulin. Cancer Res 53:4573–4581
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Fingleton B (2003) Matrix metalloproteinase inhibitors for cancer therapy: the current situation and future prospects. Expert Opin Ther Targets 7:385–397
Font A, Murias A, Garcia Arroyo FR, Martin C, Areal J, Sanchez JJ, Santiago JA, Constenla M, Saladie JM, Rosell R (2005) Sequential mitoxantrone/prednisone followed by docetaxel/estramustine in patients with hormone refractory metastatic prostate cancer: results of a phase II study. Ann Oncol 16:419–424
Forget MA, Desrosiers RR, Beliveau R (1999) Physiological roles of matrix metalloproteinases: implications for tumor growth and metastasis. Can J Physiol Pharmacol 77:465–480
Gilligan T, Kantoff PW (2002) Chemotherapy for prostate cancer. Urology 60:94–100
Handsley MM, Edwards DR (2005) Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer 115:849–860
Hidalgo M, Eckhardt SG (2001) Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 93:178–193
Himelstein BP, Canete-Soler R, Bernhard EJ, Dilks DW, Muschel RJ (1994) Metalloproteinases in tumor progression: the contribution of MMP-9. Invasion Metastasis 14:246–258
Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ (1998) Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95:365–377
Hudes G, Einhorn L, Ross E, Balsham A, Loehrer P, Ramsey H, Sprandio J, Entmacher M, Dugan W, Ansari R, Monaco F, Hanna M, Roth B (1999) Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormone-refractory prostate cancer: a Hoosier Oncology Group and Fox Chase Network phase III trial. J Clin Oncol 17:3160–3166
Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S (1998) Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 58:1048–1051
Kahari VM, Saarialho-Kere U (1999) Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med 31:34–45
Kreis W, Budman DR, Calabro A (1997) Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines. Br J Urol 79:196–202
Kreis W, Budman DR, Fetten J, Gonzales AL, Barile B, Vinciguerra V (1999) Phase I trial of the combination of daily estramustine phosphate and intermittent docetaxel in patients with metastatic hormone refractory prostate carcinoma. Ann Oncol 10:33–38
Kruger A, Soeltl R, Sopov I, Kopitz C, Arlt M, Magdolen V, Harbeck N, Gansbacher B, Schmitt M (2001) Hydroxamate-type matrix metalloproteinase inhibitor batimastat promotes liver metastasis. Cancer Res 61:1272–1275
Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic JM (2002) Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol 161:1247–1253
Lein M, Jung K, Le DK, Hasan T, Ortel B, Borchert D, Winkelmann B, Schnorr D, Loenings SA (2000) Synthetic inhibitor of matrix metalloproteinases (batimastat) reduces prostate cancer growth in an orthotopic rat model. Prostate 43:77–82
Lein M, Jung K, Ortel B, Stephan C, Rothaug W, Juchem R, Johannsen M, Deger S, Schnorr D, Loening S, Krell HW (2002) The new synthetic matrix metalloproteinase inhibitor (Roche 28-2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene 21:2089–2096
Lucia MS, Bostwick DG, Bosland M, Cockett AT, Knapp DW, Leav I, Pollard M, Rinker-Schaeffer C, Shirai T, Watkins BA (1998) Workgroup I: rodent models of prostate cancer. Prostate 36:49–55
Lynch CC, Matrisian LM (2002) Matrix metalloproteinases in tumor-host cell communication. Differentiation 70:561–573
Maquoi E, Sounni NE, Devy L, Olivier F, Frankenne F, Krell HW, Grams F, Foidart JM, Noel A (2004) Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res 10:4038–4047
Oh WK, Hagmann E, Manola J, George DJ, Gilligan TD, Jacobson JO, Smith MR, Kaufman DS, Kantoff PW (2005) A phase I study of estramustine, weekly docetaxel, and carboplatin chemotherapy in patients with hormone-refractory prostate cancer. Clin Cancer Res 11:284–289
Paparel P, Curiel L, Chesnais S, Ecochard R, Chapelon JY, Gelet A (2005) Synergistic inhibitory effect of high-intensity focused ultrasound combined with chemotherapy on Dunning adenocarcinoma. BJU Int 95:881–885
Petrylak DP (2002) Chemotherapy for androgen-independent prostate cancer. Semin Urol Oncol 20:31–35
Petrylak DP (2005) The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology 65:3–7
Pienta KJ, Lehr JE (1993) Inhibition of prostate cancer growth by estramustine and etoposide: evidence for interaction at the nuclear matrix. J Urol 149:1622–1625
Pienta KJ, Redman B, Hussain M, Cummings G, Esper PS, Appel C, Flaherty LE (1994) Phase II evaluation of oral estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol 12:2005–2012
Savarese DM, Halabi S, Hars V, Akerley WL, Taplin ME, Godley PA, Hussain A, Small EJ, Vogelzang NJ (2001) Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. Cancer and Leukemia Group B. J Clin Oncol 19:2509–2516
Sinibaldi VJ, Carducci MA, Moore-Cooper S, Laufer M, Zahurak M, Eisenberger MA (2002) Phase II evaluation of docetaxel plus one-day oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer 94:1457–1465
Smith DC, Dunn RL, Strawderman MS, Pienta KJ (1998) Change in serum prostate-specific antigen as a marker of response to cytotoxic therapy for hormone-refractory prostate cancer. J Clin Oncol 16:1835–1843
Sumiyoshi Y, Hashine K, Nakatsuzi H, Yamashita Y, Karashima T (2000) Oral estramustine phosphate and oral etoposide for the treatment of hormone-refractory prostate cancer. Int J Urol 7:243–247
Westermarck J, Kahari VM (1999) Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13:781–792
Zervos EE, Norman JG, Gower WR, Franz MG, Rosemurgy AS (1997) Matrix metalloproteinase inhibition attenuates human pancreatic cancer growth in vitro and decreases mortality and tumorigenesis in vivo. J Surg Res 69:367–371
Acknowledgments
The authors thank Sabine Becker, Silke Klotzek, and Ines Baumert for valuable technical assistance. We thank Roche Diagnostics GmbH, Pharma Research, Penzberg, Germany for providing Ro 28-2653. The study contains parts of the thesis of W.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abramjuk, C., Lein, M., Rothaug, W. et al. Enhanced inhibitory effect of the matrix metalloproteinase inhibitor Ro 28-2653 in combination with estramustine and etoposide on the prostate carcinoma in the rat Dunning orthotopic tumor model. Cancer Chemother Pharmacol 59, 275–282 (2007). https://doi.org/10.1007/s00280-006-0269-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0269-7