Abstract
Background
PEFG regimen (cisplatin and epirubicin 40 mg/m2 day 1, gemcitabine 600 mg/m2 days 1 and 8, 5-fluorouracil (FU) 200 mg/m2/day continuous infusion) significantly improved the outcome of patients with advanced pancreatic adenocarcinoma (PA) with respect to standard gemcitabine in a previous phase III trial. This regimen was subsequently modified in a dose-finding study by increasing dose intensity of cisplatin and epirubicin (both at 30 mg/m2 every 14 days) and of gemcitabine (at 800 mg/m2 every 14 days). Results of a consecutive series treated by dose-intense PEFG regimen are herewith reported.
Material and methods
Dose-intense PEFG was administered to chemotherapy-naive patients with stages III–IV PA, < 75 years, performance status (PS) > 50, till progressive disease or for a maximum of 6 months.
Results
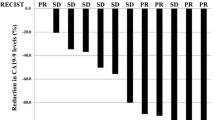
Between January 2004 and June 2005, 49 (31 or 63% metastatic) patients, median age 62 years, median PS 80, were treated with dose-intense PEFG. Partial response and stable disease was observed in 24 (49%) and 16 (33%) patients, respectively; 31 patients were progression-free at 6 months (PFS-6 = 63%). Median survival was 10.5 months and 1-year overall survival (OS) was 48% (95% confidence interval: 33–61%). Main grade 3–4 toxicity was: neutropenia in 26% of patients, stomatitis and fatigue in 8%, anaemia, diarrhoea, nausea/vomit in 6%, febrile neutropenia and thrombocytopaenia in 4%, hand-foot syndrome in 2%.
Conclusion
When compared with 84 patients treated by classical PEFG at the same institution, dose-intense PEFG was not inferior in terms of PFS-6 (63 versus 57%), 1-year OS (48 versus 42%) and response rate (49 versus 49%); it allowed to increase dose intensity for gemcitabine by 32%, for cisplatin and epirubicin by 36% (FU reduced by 3%), to significantly reduce grade 3–4 hematological toxicity (neutropenia: 26 versus 86%; P < 0.00001; thrombocytopaenia: 4 versus 58%; P < 0.00001) and to reduce by one-third the number of outpatient accesses. The new PEFG schedule appears more suitable for clinical use and should be preferred as a basis for further development of therapeutic strategies against pancreatic cancer.

Similar content being viewed by others
References
Moore MJ, Goldstein D, Hamm J et al (2005) Erlotinib improves survival when added to gemcitabine in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trial Group (NCIC-CTG). In: Proceedings of the 2005 Gastrointestinal Cancers Symposium, Hollywood, FL, USA, 3: abstr 77, 27–29 Jan 2005
Cunningham D, Chau I, Stocken D et al (2005) Phase III randomised comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. Eur J Cancer 3:12 (abstr PS 11)
Reni M, Cordio S, Milandri C et al (2005) Gemcitabine versus cisplatin, epirubicin, 5-fluorouracil, gemcitabine in advanced pancreatic cancer: a phase III trial. Lancet Oncol 6:369–376
Reni M, Passoni P, Panucci MG et al (2001) Definitive results of a phase II trial of PEF-G (cisplatin, epirubicin, 5-fluorouracil continuous infusion, gemcitabine) in stage IV pancreatic adenocarcinoma. J Clin Oncol 19:2679–2686
Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al (1997) Improvement in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A et al (2003) Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 21:3296–3302
Rocha Lima CMS, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA et al (2004) Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 22:3776–3783
Bramhall SR, Schultz J, Nemunaitis J, Brown PD, Baillet M, Buckels JAC (2002) A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 87:161–167
Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA, for the Marimastat Pancreatic Cancer Study Group (2001) Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 19:3477–3455
Heinemann V, Quietzsch D, Gieseler F, Gonnermann N, Schonekas H, Rost A, et al. (2003) A phase III trial comparing gemcitabine plus cisplatin vs. gemcitabine alone in advanced pancreatic adenocarcinoma. Proc Am Soc Clin Oncol 22 (abstr 1003)
Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A et al (2004) Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 22:1430–1438
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taı¨eb J, Faroux R, Lepere C, de Gramont A (2005) Gemcitabine in combination with Oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516
O’Reilly EM, Abou-Alfa GK, Letorneau R, Harker WG, Modiano M, Hurwitz H et al (2004) A randomized phase III trial of DX-8951f (exatecan mesylate) and gemcitabine vs. gemcitabine alone in advanced pancreatic cancer. Proc Am Soc Clin Oncol 23 (abstr 4006)
Oettle H, Richards DA, Ramanathan R, Van Laethem JL, Peeters M, Fuchs M et al (2005) A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 16:1639–1645
Cheverton P, Friess H, Andras C et al (2004) Phase III results of exatecan (DX-8951f) versus gemcitabine (Gem) in chemotherapy-naive patients with advanced pancreatic cancer (APC). Proc Am Soc Clin Oncol 23:314s (abstr 4005)
Dell’Oro S, Reni M, Bonetto E et al (2004) Intensified PEFG regimen in stage III-IV pancreatic adenocarcinoma. Ann Oncol 15:242 (abstr 919)
Therasse P, Arbuck SG, Eisenhaur EA, Wanders J, Kaplan RS, Rubinstein L et al (2004) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH (1990) Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8:147–159
Reni M, Passoni P, Bonetto E et al (2005) Final results of a prospective trial of a PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen followed by radiotherapy after curative surgery for pancreatic adenocarcinoma. Oncology 68:239–245
Topham C, Glees J, Coombs R et al (1993) Comparison of single agent epirubicin and 5-FU/epirubicin/mitomycin in patients with advanced adenocarcinoma of the pancreas. Oncology 50:78–83
Wils JA, Bleiberg H, Blijham G et al (1985) Phase II study of epirubicin in advanced adenocarcinoma of the pancreas. Eur J Cancer 21:191–194
Wils JA, Kok T, Wagener DJ et al (1993) Activity of cisplatin in adenocarcinoma of the pancreas. Eur J Cancer 29A:203–204
Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T et al (2002) Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 20:3130–3136
Hidalgo M, Castellano D, Paz-Ares L et al (1999) Phase II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol 17:585–592
Rothman H, Cantrell JE, Lokich J et al (1991) Continuous infusion 5-fluorouracil plus weekly cisplatin for pancreatic adenocarcinoma: a Mid-Atlantic Oncology Program study. Cancer 68:264–268
Nicolson M, Webb A, Cunningham D et al (1995) Cisplatin and protracted venous infusion 5-fluorouracil (CF)––good symptom relief with low toxicity in advanced pancreatic carcinoma. Ann Oncol 6:801–804
Rougier P, Zarba JJ, Ducreux M et al (1993) Phase II study of cisplatin and 120-hour continuous infusion of 5-fluorouracil in patients with advanced pancreatic adenocarcinoma. Ann Oncol 4:333–336
Scheitauer W, Kornek GV, Raderer M et al (1999) Phase II trial of gemcitabine, epirubicin and granulocyte colony-stimulating factor in patients with advanced pancreatic adenocarcinoma. Br J Cancer 80:1797–1802
Heinemann V, Wilke H, Mergenthaler H-G et al (2000) Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol 11:1399–1403
Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R et al (2002) A randomised trial comparing 5-FU with 5-FU plus cisplatin in advanced pancreatic carcinoma. Ann Oncol 13:1185–1191
Evans TRJ, Lofts FJ, Mensi JL et al (1996) A phase II study of continuous infusion 5-fluorouracil with cisplatin and epirubicin in inoperable pancreatic cancer. Br J Cancer 73:1260–1264
El-Rayes BF, Zalupski MM, Shields AF et al (2003) Phase II study of gemcitabine, cisplatin, and infusional fluorouracil in advanced pancreatic cancer. J Clin Oncol 21:2920–2925
Reni M, Bonetto E, Cordio S, Passoni P, Milandri C, Cereda S, et al. (2006) Quality of life assessment in advanced pancreatic adenocarcinoma: results from a phase III randomized trial. Pancreatology (in press)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reni, M., Cereda, S., Bonetto, E. et al. Dose-intense PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 59, 361–367 (2007). https://doi.org/10.1007/s00280-006-0277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0277-7