Abstract
Purpose
We developed a laboratory based regimen called GTX which induces synergistic apoptosis in human pancreatic cancer cells. This retrospective review summarizes our clinical experience with GTX in an initial group of 35 patients; 66% untreated and 34% failed prior therapies.
Methods
All patients treated with GTX for metastatic pancreatic cancer, prior to initiation of a prospective phase II trial of GTX were assessed and followed until death. GTX consisted of capecitabine (X), 750 mg/m2 p.o. BID on days 1–14, gemcitabine (G) (750 mg/m2) over 75 min and docetaxel (T) (30 mg/m2) on days 4 and 11. Thus one cycle of GTX was 14 days with 7 days off for a 21 day cycle. Tumor assessments were repeated every 3 cycles.
Results
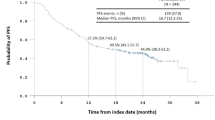
All 35 patients had metastatic pancreatic cancer (94% liver, 6% lung sites). Grade 3–4 hematological toxicities were: leukopenia and thrombocytopenia—both 14%, and anemia 9%, respectively. The overall response rate of all 35 patients treated with GTX (from 0.5 cycles onward) was 29% (CR/PR) by WHO criteria, and 31% had a minor response or stable disease (MR, SD). At the metastatic sites for the 35 patients, there were 9% complete (CR) and 31% partial (PR) responses (total 40%). For the 31 patients who had their primary tumor (4 patients had a prior Whipple resection), there were 13% CR and 19% PR for a response rate of 32% at the primary tumor site. Overall median progression free survival of responders was 6.3 months (95% C.I. 4.4–10.4 months) and median survival was 11.2 months (95% C.I. 8.1–15.1 months). Survival after initiation of GTX at 12, 18, 24 and 30 months was 43, 29, 20, and 11%, respectively.
Conclusion
Our retrospective review suggests that GTX has potential as a regimen for untreated and treated metastatic pancreatic cancer.


Similar content being viewed by others
References
Jemal A, Murray T, Samuels A (2003) Cancer statistics, 2003. CA-Cancer J Clin 53:5–26
Burris HA, Moore MJ, Anderson J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Onc 15(6):2403–2413
Brand R, Capadano M, Tempero M (1997) A phase I trial of weekly gemcitabine administered as a prolonged infusion in patients with pancreatic cancer and other solid tumors. Invest New Drugs 15(4):331–341
Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J (2003) Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Onc 21(18):3402–3408
Moore M, Goldstein D, Hamm J et al (2005) Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. J Clin Oncol 23:1s, (Suppl, Abstr 1)
Cunningham D, Chau I, Stocken D, Barletta E, Moscetti L, Recchia F et al Phase III randomised comparison of gemcitabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. Eur J Cancer Suppl 4, Abstr. PS 11
Fine RL, Fogelman DR, Sherman W et al (2003) The GTX regimen: A biochemically synergistic combination for advanced pancreatic cancer (PC). Proc Am Soc Clin Onc Abstr #1129
Fine RL, Fogelman DR, Schreibman S, Guba S, Sharma J, Shapiro G (2004) GTX chemotherapy for metastatic pancreatic cancer: Response, survival, and toxicity data. J Clin Oncol 22:381s suppl. Abstr #4271
Sherman WH, Fine RL (2001) Combination gemcitabine and docetaxel in advanced adenocarcinoma of the pancreas. Oncology 60(4):316–321
Hess V, Salzberg M, Borner M et al (2003) Combining capecitabine and gemcitabine in patients with advanced pancreatic carcinoma: A phase I/II trial. J Clin Oncol 21(1):66–68
Fogelman DR, Sherman W, Schreibman S, Fine RL (2003) Effective salvage chemotherapy with minimal toxicity for relapsed, advanced pancreatic cancer. Proc Am Soc Clin Onc Abstr #1517
Fogelman D, Fine RL, Schreibman S (2004) Effective salvage therapy (T-GX) for pancreatic cancer patients after chemotherapy with GTX. J Clin Oncol 22:380s suppl. Abstr #4268
Khorana A, Fine RL (2004) Pancreatic cancer and thromboembolic disease. Lancet Oncol 5(11):655–663
Poplin E, Levy D, Berlin J et al (2006) Phase III trial of gemcitabine (30-minute infusion) versus gemcitabine (fixed-dose-rate infusion [FDR]) versus gemcitabine + oxaliplatin(GEMOX) in patients with advanced pancreatic cancer (E6201). J Clin Oncol 24(18):2006, LBA 4004
Cartwright TH, Cohn A, Varkey JA et al (2002) Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol 20(1):160–164
Stathopoulos GP, Syrigos K, Polyzos A et al (2004) Front-line treatment of inoperable or metastatic pancreatic cancer with gemcitabine and capecitabine: an intergroup, multicenter, phase II study. Ann Oncol 15:224–229
Hermann R, Bodoky G, Ruhstaller T et al (2005) Gemcitabine (G) plus capecitabine versus G alone in advanced/metastatic pancreatic cancer. A randomized phase III study of the SAKK and CECOG groups, Proc Am Soc Clin Oncol Abst #4010
Rougier P, Adenis A, Dureux M et al (2000) A phase II study: Docetaxel as first line chemotherapy for advanced pancreatic adenocarcinoma. Eur J Cancer 36:1016–1025
Androulakis N, Kourousis C, Dimopoulos M (1999) Treatment of pancreatic cancer with docetaxel and granulocyte colony-stimulating factor: a multicenter phase II trial. J Clin Oncol 17:1779–1785
Schneider BP, Ganjoo KN, Seitz DE et al (2003) Phase II study of gemcitabine plus docetaxel in advanced pancreatic cancer: a Hoosier Oncology Group study. Oncology 65(3):218–223
Louvet C et al (2004) Gemcitabine versus GEMOX (gemcitabine + oxaliplatin) in non resectable pancreatic adenocarcinoma : Final results of the GERCOR /GISCAD Intergroup Phase III. J Clin Oncol 22:315s suppl, abstr #4008
Heineman V, Quietzsch D, Gieseler F (2003) A phase III trial comparing gemcitabine plus cisplatin vs. gemcitabine alone in advanced pancreatic carcinoma, Proc Am Soc Clin Oncol Abst #1003
Rocha Lima CM, Rotche R, Jeffrey M et al (2003) A randomized phase 3 study comparing efficacy and safety of gemcitabine (GEM) and irinotecan (I), to GEM alone in patients (pts) with locally advanced or metastatic pancreatic cancer who have not received prior systemic therapy. Proc Am Soc Clin Onc Abst #1005
Kozuch P, Grossbard ML, Barzdins A et al (2001) Irinotecan Combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and noncrossresistant treatment for chemotherapy refractory pancreatic cancer. Oncologist 6(6):488–495
El-Rayes BF, Zalupski MM, Shields AF et al (2003) Phase II study of gemcitabine, cisplatin, and infusional fluorouracil in advanced pancreatic cancer. J Clin Oncol 15(1):2920–2925
Araneo M, Bruckner HW, Grossbard ML et al (2003) Biweekly low-dose sequential gemcitabine, 5-fluorouracil, leucovorin and cisplatin (GFP): A highly active novel therapy for metastatic adenocarcinoma of the exocrine pancreas. Cancer Invest 21(4):489–496
CALGB News, http://www.calgb.org/index.php?action=fullnews&id = 28; or http://www.calgb.org website
Kindler HL, Bylow KA, Hochster HS et al (2006) A randomized phase III study of bevacizumab (B) and gemcitabine (G) plus cetuximab (C) or erlotinib (E) in patients (pts) with advanced pancreatic cancer (PC): a preliminary analysis. J Clin Onc 24(18S):A4040
Berlin JD, Adak S, Vaughn DJ et al (2000) A phase II study of gemcitabine and 5-fluorouracil in metastatic pancreatic cancer: an Eastern Cooperative Oncology Group study (E3296). Oncology 58:215–218
Berlin JD, Catalano P, Thomas J et al (2002) Phase III study of gemcitabine with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Reiss H, Helm A, Niedergethmann M et al (2005) Randomized, prospective multicenter phase III trial of gemcitabine, 5FU, folinic acid versus gemcitabine alone in patients with advanced pancreatic cancer. Proc Am Soc Clin Oncol Abst #4009
Hidalgo M, Castellano D, Paz-Ares L et al (1999) Phase I-II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol 17(2):585–592
Fine RL, Fogelman DR, Sherman W et al (2006) Gemcitabine, Docetaxel, and Capecitabine (GTX) in the treatment of metastatic pancreatic cancer (PC): A prospective phase II Study. Proc Am Soc Clin Oncol Abst #14024
Sawada N, Ishikawa T, Fukase Y et al (1998) Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/docetaxel in human cancer xenografts. Clin Cancer Res Suppl 4(4):1013–1019
Fogelman DR, Chen J, Chabot J, Allendorf J, Schreibman S, Ennis R, Fine RL (2004) The evolution of adjuvant and neoadjuvant chemotherapy and radiation for advanced pancreatic cancer: From 5-FU to GTX. Surg Oncol Clin No Am 13:711–735
Acknowledgments
We wish to thank the Marcove and Manelski Family Foundations, Susan Grant Kaplansky Memorial Fund and the Herbert Irving Scholar Award for support of these clinical and laboratory studies to RLF. The clinical expertise of our Data Collector, Cara DeRosa, and Research Nurse, Kyung Chu, was invaluable to this report. We also thank Susan Bronson and Gina Egan for their excellent assistance in the preparation of this manuscript. This paper is dedicated to the memory and courage of Denis Manelski who was one of the first patients to receive GTX and lived 3 years with metastatic (liver) pancreatic cancer.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00280-007-0569-6
Rights and permissions
About this article
Cite this article
Fine, R.L., Fogelman, D.R., Schreibman, S.M. et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol 61, 167–175 (2008). https://doi.org/10.1007/s00280-007-0473-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0473-0