Abstract
Purpose
To define dose limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of capecitabine with fixed-dose rate (FDR) gemcitabine.
Methods
Eligible adults (advanced solid tumor; performance status ≤2) received capecitabine 500 mg/m2 PO BID days 1–14 and FDR gemcitabine (400–1,000 mg/m2 escalated by 200 mg/m2 increments) at 10 mg/m2/min days 1 and 8 on a 21-day cycle. A traditional 3 + 3 cohort design was used to determine the MTD.
Results
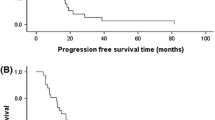
Thirty patients (median age 59 years) were enrolled. The predominant grade ≥3 toxicity was myelosuppression, particularly neutropenia. At dose level 4 (1,000 mg/m2 gemcitabine), two out of five evaluable patients had a DLT (grade 4 neutropenia ≥7 days). At dose level 3 (800 mg/m2 gemcitabine), one patient had a DLT (grade 3 neutropenia ≥7 days) among six evaluable patients. Therefore, the MTD and recommended phase II dose was designated as capecitabine 500 mg/m2 PO BID days 1–14 with 800 mg/m2 FDR gemcitabine days 1 and 8 infused at 10 mg/m2 per min on a 21-day cycle. Partial responses occurred in pretreated patients with esophageal, renal cell and bladder carcinomas.
Conclusions
This regimen was well tolerated and may deserve evaluation in advanced gastrointestinal and genitourinary carcinomas.
Similar content being viewed by others
References
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN et al (1991) A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 9:491–498
Benekli M, Yildiz R, Uner A, Er O, Yamac D, Alkis N, Coskun U, Camci C, Buyukberber S (2007) Gemcitabine plus capecitabine combination in metastatic breast cancer patients previously treated with anthracyclines and taxanes. Oncology 72:308–313
Boeck S, Hoehler T, Seipelt G, Mahlberg R, Wein A, Hochhaus A, Boeck HP, Schmid B, Kettner E, Stauch M, Lordick F, Ko Y, Geissler M, Schoppmeyer K, Kojouharoff G, Golf A, Neugebauer S, Heinemann V (2008) Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 19:340–347
Grunewald R, Kantarjian H, Du M, Faucher K, Tarassoff P, Plunkett W (1992) Gemcitabine in leukemia: a phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol 10:406–413
Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Kohne CH, Mingrone W, Stemmer SM, Tamas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25:2212–2217
Hess V, Salzberg M, Borner M, Morant R, Roth AD, Ludwig C, Herrmann R (2003) Combining capecitabine and gemcitabine in patients with advanced pancreatic carcinoma: a phase I/II trial. J Clin Oncol 21:66–68
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23:2332–2338
Mackean M, Planting A, Twelves C, Schellens J, Allman D, Osterwalder B, Reigner B, Griffin T, Kaye S, Verweij J (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16:2977–2985
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Park BB, Park JO, Lee HR, Lee J, Choi DW, Choi SH, Heo JS, Lee JK, Lee KT, Lim do H, Park YS, Lim HY, Kang WK, Park K (2007) A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 60:489–494
Poplin E, Levy D, Berlin J, Rothenberg M, Cella D, Mitchell E, Alberts S, Benson A (2006) Phase III trial of gemcitabine (30-minute infusion) versus gemcitabine (fixed-dose-rate infusion[FDR]) versus gemcitabine + oxaliplatin(GEMOX) in patients with advanced pancreatic cancer (E6201). Proc Am Soc Clin Oncol 24:4004
Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ (2007) Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 110:1307–1312
Rini BI, Weinberg V, Small EJ (2005) A phase I trial of fixed dose rate gemcitabine and capecitabine in metastatic renal cell carcinoma. Cancer 103:553–558
Santini D, Vincenzi B, Masi G, Catalano V, Virzi V, Fontana A, Vasile E, Intagliata S, Catalano G, Falcone A, Tonini G (2008) Phase II capecitabine and gemcitabine fixed dose rate (FDR) in patients with advanced pancreatic cancer. J Clin Oncol 26, May 20 Suppl, Abstract 15596
Santini D, Vincenzi B, Masi G, Catalano V, Virzi V, Vasile E, Intagliata S, Catalano G, Falcone A, Tonini G (2008) Fixed dose rate (FDR) gemcitabine (G) and capecitabine (C) in patients with metastatic biliary tract cancer (BTC): phase II trial. 2008 Gastrointestinal Cancers Symposium, Abstract No. 223
Santini D, Virzi V, Vincenzi B, Rocci L, Leoni V, Tonini G (2007) A phase I trial of fixed dose rate gemcitabine plus capecitabine in metastatic cancer patients. Ann Oncol 18:576–580
Schilsky RL, Bertucci D, Vogelzang NJ, Kindler HL, Ratain MJ (2002) Dose-escalating study of capecitabine plus gemcitabine combination therapy in patients with advanced cancer. J Clin Oncol 20:582–587
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45:291–297
Schulz L SA, Wilmanns W et al (1998) Synergistic interaction of gemcitabine and 5-fluorouracil in colon cancer cells. Proc Am Soc Clin Oncol 17:965
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147
Stadler WM, Halabi S, Rini B, Ernstoff MS, Davila E, Picus J, Barrier R, Small EJ (2006) A phase II study of gemcitabine and capecitabine in metastatic renal cancer: a report of Cancer and Leukemia Group B protocol 90008. Cancer 107:1273–1279
Storer BE (1989) Design and analysis of phase I clinical trials. Biometrics 45:925–937
Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J (2003) Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21:3402–3408
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Waters JS, Moss C, Pyle L, James M, Hackett S, A’Hern R, Gore M, Eisen T (2004) Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. Br J Cancer 91:1763–1768
Acknowledgments
We thank our patients and their families for their participation in this study. We thank the nurses and research specialists of the University of Wisconsin Phase I Program for their devotion to these patients and this study. The study was partially supported by Roche. We acknowledge support from National Institutes of Health grant T32 CA009614 Physician Scientist Training in Cancer Medicine (Dr. Attia).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The results of this research appeared as abstract ID 13509 at the 2008 American Association of Clinical Oncology meeting in Chicago, IL, USA.
Rights and permissions
About this article
Cite this article
Attia, S., Morgan-Meadows, S., Holen, K.D. et al. Dose-escalation study of fixed-dose rate gemcitabine combined with capecitabine in advanced solid malignancies. Cancer Chemother Pharmacol 64, 45–51 (2009). https://doi.org/10.1007/s00280-008-0844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0844-1