Abstract
Purpose
Paclitaxel is an effective therapy for patients with solid tumors. While the albumin-bound formulation eliminates the hypersensitivity reaction caused by the Cremaphor solvent, significant peripheral neuropathy persists when given over the standard 30-min infusion time. We sought to determine if the incidence and severity of peripheral neuropathy could be reduced when the infusion time is lengthened to 2-h.
Methods
This was an open-label, single-arm, phase 2 study of albumin-bound paclitaxel given over 2 h. Twenty-five patients with advanced non-small-cell lung cancer were enrolled to determine whether the longer infusion reduced the severity of neuropathy compared to data from an earlier cohort of 40 similar patients treated over 30 min Patients received 125 mg/m2 of albumin-bound paclitaxel IV over 2 h without premedication on days 1, 8, and 15 of a 28-day cycle. Radiologic assessment was performed every 8 weeks.
Results
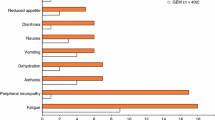
There was a significant 0.45 grade decrease in average peripheral neuropathy experienced by patients in the 2-h group versus the 30-min group (90% CI, 0.03-0.87). There was, in addition, a significant decrease in grade ≥2 peripheral neuropathy in patients treated over 2 h versus 30 min (28% vs. 55%, 2-sided P = 0.04). A decrease in grade ≥2 neutropenia (20% vs. 48%, 2-sided P = 0.07) was also observed. The median survival, 11 months, was the same for both groups.
Conclusion
Increasing the infusion time of albumin-bound paclitaxel from 30 min to 2 h resulted in a significant reduction in both average and grade ≥2 peripheral neuropathy without affecting survival.

Similar content being viewed by others
References
Blum JL, Savin MA, Edelman G, Pippen JE, Robert N, Sandbach J, Carrasco S, O’Shaughnessy JA (2004) Long term disease control in taxane-refractory metastatic breast cancer treated with nab paclitaxel. J Clin Oncol (Meeting Abstracts) 22(14S):abstr 543
Eisenhauer E, ten Bokkel Huinink W, Swenerton K, Gianni L, Myles J, van der Burg M, Kerr I, Vermorken J, Buser K, Colombo N (1994) European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 12:2654–2666
Freilich R, Balmaceda C, Seidman A (1996) Motor neuropathy due to docetaxel and paclitaxel. Neurology 47:115–118
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, Hawkins MJ (2006) Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol 17:1263–1268
Ibrahim N, Samuels B, Page R, Guthrie G, Hortobagyi G (2002) Nanoparticle paclitaxel (ABI-007) in metastatic breast cancer (MBC): Efficacy and evidence of dose-dependent activity in two multicenter phase II studies.In: Proceedings of American Society Clinical Oncology 21:abstr 209
Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, Nair MK, Bhar P, Desai N, Hortobagyi GN (2005) Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol 23:6019–6026
Lee JJ, Swain SM (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 24:1633–1642
Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, Mross K (2005) Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin Cancer Res 11:4843–4850
O’Shaughnessy J BJ, Sandbach J (2004) Weekly nanoparticle albumin paclitaxel (Abraxane) results in long-term disease control in patients with taxane-refractory metastatic breast cancer. In: Proceedings of 27th annual San Antonio breast cancer symposium, San Antonio, TX. abstr 1070
Peretz T, Sulkes A, Chollet P, Gelmon K, Paridaens R, Gorbonuva V, Catimel G, Kuhnle H, WtB Huinink, Khayat D, Ditrich C, Klaassen U, Bergh J, Wilking N, Nabholtz JM, Calabresi F, Tubiana-Hulin M, Chazard M, Gallant G, Diergarten K, Westberg R, Bogaert J, Renard J, Weil C (1995) A multicenter, randomized study of two schedules of paclitaxel (PTX) in patients with advanced breast cancer (ABC). Eur J Cancer 31:75–75
Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, Lopez P, Anderson H, Gustafson N, Jeynes A, Gallant G, Washington T, Thatcher N (2000) Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 92:1074–1080
Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ, Kris MG (2008) Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 26:639–643
Roszkowski K, Pluzanska A, Krzakowski M, Smith AP, Saigi E, Aasebo U, Parisi A, Pham Tran N, Olivares R, Berille J (2000) A multicenter, randomized, phase III study of docetaxel plus best supportive care versus best supportive care in chemotherapy-naive patients with metastatic or non-resectable localized non-small-cell lung cancer (NSCLC). Lung Cancer 27:145–157
Roytta M, Raine S (1986) Taxol induced neuropathy: chronic effects of local injection. J Neurocytol 15:483–496
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all her-2 overexpressors and random assignment to trastuzumab or not in her-2 non-overexpressors: final results of cancer and leukemia group b protocol 9840. J Clin Oncol 26:1642–1649
Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH, Shibata HR, Baez L, DeFusco PA, Davila E, Tipping SJ, Bearden JD, Thirlwell MP (1999) Randomized trial of 3 h versus 24-h infusion of high-dose paclitaxel in patients with metastatic or locally advanced breast cancer: national surgical adjuvant breast and bowel project protocol b-26. J Clin Oncol 17:3403–3411
Socinski M, Bondarenko I, Karaseva N, Makhson A, Vynnychenko I, Okamoto I, Hon J (2010) Results of a randomized, phase III trial of nab-paclitaxel (nab-P) and carboplatin (C) compared with Cremophor-based paclitaxel (P) and carboplatin as first-line therapy in advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 28:abstr 7511
Socinski MA, Manikhas GM, Stroyakovsky DL, Makhson AN, Cheporov SV, Orlov SV, Yablonsky PK, Bhar PH, Iglesias J (2010) A dose finding study of weekly and every 3-week nab-paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol 5:852–861
Stokes M, Davis C, Koch G (2000) Categorical data analysis using the sas system, 2nd edn. SAS Institute and Wiley, Cary
Acknowledgments
Grant support is given by Abraxis BioScience, Los Angeles, CA.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paik, P.K., James, L.P., Riely, G.J. et al. A phase 2 study of weekly albumin-bound paclitaxel (Abraxane®) given as a two-hour infusion. Cancer Chemother Pharmacol 68, 1331–1337 (2011). https://doi.org/10.1007/s00280-011-1621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1621-0