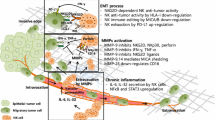
Abstract
Natural killer (NK) cells are the primary effector cells of the innate immune system and have a well-established role in tumor rejection in a variety of spontaneous and induced cancer models. NK cell function is regulated by a complex balance of inhibitory and activating signals that allow them to selectively target and kill cells that display an abnormal pattern of cell surface molecules, while leaving normal healthy cells unharmed. In this review we discuss NK cell function, the role of NK cells in cancer therapies, the emerging concept of bi-directional cross-talk between NK cells and dendritic cells, and the implications of these interactions for tumor immunotherapy.

Similar content being viewed by others
References
Ballas ZK, Turner JM, Turner DA, et al (1990) A patient with simultaneous absence of “classical” natural killer cells (CD3–, CD16+, and NKH1+) and expansion of CD3+, CD4–, CD8–, NKH1+ subset. J Allergy Clin Immunol 85:453
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245
Bauer S, Groh V, Wu J, et al (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727
Bennett IM, Zatsepina O, Zamai L, et al (1996) Definition of a natural killer NKR-P1A+/CD56–/CD16– functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med, 184:1845
Borrego F, Kabat J, Kim DK, et al (2002) Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol 38:637
Brady J, Hayakawa Y, Smyth MJ, et al (2004) IL-21 induces the functional maturation of murine NK cells. J Immunol 172:2048
Buentke E, Heffler LC, Wilson JL, et al (2002) Natural killer and dendritic cell contact in lesional atopic dermatitis skin—Malassezia-influenced cell interaction. J Invest Dermatol 119:850
Campbell JJ, Qin S, Unutmaz D, et al (2001) Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 166:6477
Cerwenka A, Baron JL, Lanier LL (2001) Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA 98:11521
Cerwenka A, Lanier LL (2001) Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev 181:158
Cerwenka A, Lanier LL (2001) Natural killer cells, viruses and cancer. Nat Rev Immunol 1:41
Chalifour A, Jeannin P, Gauchat JF, et al (2004) Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 104:1778
Colucci F, Caligiuri MA, Di Santo JP (2003) What does it take to make a natural killer? Nat Rev Immunol 3:413
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633
Cooper MA, Fehniger TA, Fuchs A, et al (2004) NK cell and DC interactions. Trends Immunol 25:47
Cretney E, Takeda K, Yagita H, et al (2002) Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 168:1356
Cui Z, Willingham MC, Hicks AM, et al (2003) Spontaneous regression of advanced cancer: identification of a unique genetically determined, age-dependent trait in mice. Proc Natl Acad Sci USA 100:6682
Davis JE, Smyth MJ, Trapani JA (2001) Granzyme A and B-deficient killer lymphocytes are defective in eliciting DNA fragmentation but retain potent in vivo anti-tumor capacity. Eur J Immunol 31:39
Della Chiesa M, Vitale M, Carlomagno S, et al (2003) The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol 33:1657
Diefenbach A, Jensen ER, Jamieson AM, et al (2001) Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413:165
Diefenbach A, Tomasello E, Lucas M, et al (2002) Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol 3:1142
Dokun AO, Kim S, Smith HR, et al (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2:951
Edwards AD, Diebold SS, Slack EM, et al (2003) Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol 33:827
Ferlazzo G, Morandi B, D’Agostino A, et al (2003) The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol 33:306
Ferlazzo G, Munz C (2004) NK cell compartments and their activation by dendritic cells. J Immunol 172:1333
Ferlazzo G, Semino C, Melioli G (2001) HLA class I molecule expression is up-regulated during maturation of dendritic cells, protecting them from natural killer cell-mediated lysis. Immunol Lett 76:37
Ferlazzo G, Thomas D, Lin SL, et al (2004) The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 172:1455
Ferlazzo G, Tsang ML, Moretta L, et al (2002) Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 195:343
Fernandez NC, Lozier A, Flament C, et al (1999) Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 5:405
Gallucci S, Lolkema M, Matzinger P (1999) Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5:1249
Gao Y, Yang W, Pan M, et al (2003) Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med 198:433
Garni-Wagner BA, Purohit A, Mathew PA, et al (1993) A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol 151:60
Geldhof AB, Raes G, Bakkus M, et al (1995) Expression of B7–1 by highly metastatic mouse T lymphomas induces optimal natural killer cell-mediated cytotoxicity. Cancer Res 55:2730
Gerosa F, Baldani-Guerra B, Nisii C, et al (2002) Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 195:327
Granucci F, Vizzardelli C, Pavelka N, et al (2001) Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol 2:882
Granucci F, Zanoni I, Pavelka N, et al (2004) A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med 200:287
Griffith TS, Chin WA, Jackson GC, et al (1998) Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 161:2833
Haliotis T, Ball JK, Dexter D, et al (1985) Spontaneous and induced primary oncogenesis in natural killer (NK)-cell-deficient beige mutant mice. Int J Cancer 35:505
Hashimoto W, Tanaka F, Robbins PD, et al (2003) Natural killer, but not natural killer T, cells play a necessary role in the promotion of an innate antitumor response induced by IL-18. Int J Cancer 103:508
Hayakawa Y, Kelly JM, Westwood JA, et al (2002) Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J Immunol 169:5377
Hayakawa Y, Screpanti V, Yagita H, et al (2004) NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol 172:123
Hayakawa Y, Takeda K, Yagita H, et al (2002) IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood 100:1728
Houchins JP, Yabe T, McSherry C, et al (1991) DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med 173:1017
Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13:95
Irmler M, Thome M, Hahne M, et al (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388:190
Kadowaki N, Ho S, Antonenko S, et al (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863
Karre K, Ljunggren HG, Piontek G, et al (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675
Kasaian MT, Whitters MJ, Carter LL, et al (2002) IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16:559
Kelly JM, Darcy PK, Markby JL, et al (2002) Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol 3:83
Kelly JM, Takeda K, Darcy PK, et al (2002) A role for IFN-gamma in primary and secondary immunity generated by NK cell-sensitive tumor-expressing CD80 in vivo. J Immunol 168:4472
Kennedy MK, Glaccum M, Brown SN, et al (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191:771
Kiessling R, Petranyi G, Klein G, et al (1975) Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer 15:933
Kim S, Iizuka K, Kang HS, et al (2002) In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 3:523
Lanier LL (1998) NK cell receptors. Annu Rev Immunol 16:359
Lanier LL, Le AM, Civin CI, et al (1986) The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 136:4480
Lian RH, Kumar V (2002) Murine natural killer cell progenitors and their requirements for development. Semin Immunol 14:453
Liao NS, Bix M, Zijlstra M, et al (1991) MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253:199
Ljunggren HG, Karre K (1985) Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med 162:1745
Loza MJ, Perussia B (2001) Final steps of natural killer cell maturation: a model for type 1-type 2 differentiation? Nat Immunol 2:917
Martin-Fontecha A, Thomsen LL, Brett S et al. (2004) Induced recruitment of NK cells to lymph nodes provides IFN-γ for Th1 priming. Nat Immunol 5:1260
McQueen KL, Parham P (2002) Variable receptors controlling activation and inhibition of NK cells. Curr Opin Immunol 14:615
Medzhitov R, Janeway CA Jr (1998) Innate immune recognition and control of adaptive immune responses. Semin Immunol 10:351
Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255
Mocikat R, Braumuller H, Gumy A, et al (2003) Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561
Moroz A, Eppolito C, Li Q, et al (2004) IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol 173:900
Moser M (2003) Dendritic cells in immunity and tolerance-do they display opposite functions? Immunity 19:5
O’Hanlon LH (2004) Natural born killers: NK cells drafted into the cancer fight. J Natl Cancer Inst 96:651
Ogasawara K, Yoshinaga SK, Lanier LL (2002) Inducible costimulator costimulates cytotoxic activity and IFN-gamma production in activated murine NK cells. J Immunol 169: 3676
Pawelec G, Da Silva P, Max H, et al (1995) Relative roles of natural killer- and T cell-mediated anti-leukemia effects in chronic myelogenous leukemia patients treated with interferon-alpha. Leuk Lymphoma 18:471
Peritt D, Robertson S, Gri G, et al (1998) Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol 161:5821
Piccioli D, Sbrana S, Melandri E, et al (2002) Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 195:335
Reis e Sousa C, Diebold SD, Edwards AD, et al (2003) Regulation of dendritic cell function by microbial stimuli. Pathol Biol (Paris) 51:67
Robertson MJ (2002) Role of chemokines in the biology of natural killer cells. J Leukoc Biol 71:173
Rosenberg SA (2000) Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am 6 Suppl 1: S2
Roth C, Carlyle JR, Takizawa H, et al (2000) Clonal acquisition of inhibitory Ly49 receptors on developing NK cells is successively restricted and regulated by stromal class I MHC. Immunity 13:143
Ruggeri L, Capanni M, Urbani E, et al (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295: 2097
Sauter B, Albert ML, Francisco L, et al (2000) Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423
Screpanti V, Wallin RP, Ljunggren HG, et al (2001) A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol 167:2068
Seya T, Akazawa T, Uehori J, et al (2003) Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res 23:4369
Shortman K, Liu YJ (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151
Silla LM, Whiteside TL, Ball ED (1995) The role of natural killer cells in the treatment of chronic myeloid leukemia. J Hematother 4:269
Sivakumar PV, Foster DC, Clegg CH (2004) Interleukin-21 is a T-helper cytokine that regulates humoral immunity and cell-mediated anti-tumour responses. Immunology 112:177
Sivori S, Falco M, Della Chiesa M, et al (2004) CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA 101:10116
Smyth MJ, Cretney E, Takeda K, et al (2001) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med 193:661
Smyth MJ, Crowe NY, Godfrey DI (2001) NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 13:459
Smyth MJ, Hayakawa Y, Takeda K, et al (2002) New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2:850
Smyth MJ, Street SE, Trapani JA (2003) Cutting edge: granzymes A and B are not essential for perforin-mediated tumor rejection. J Immunol 171:515
Smyth MJ, Takeda K, Hayakawa Y, et al (2003) Nature’s TRAIL—on a path to cancer immunotherapy. Immunity 18:1
Smyth MJ, Taniguchi M, Street SE (2000) The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol 165:2665
Smyth MJ, Thia KY, Cretney E, et al (1999) Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol 162:6658
Smyth MJ, Thia KY, Street SE, et al (2000) Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 191:661
Smyth MJ, Thia KY, Street SE, et al (2000) Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 192:755
Smyth MJ, Trapani JA (1995) Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today 16:202
Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al (1999) Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286:1957
Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97:192
Street SE, Hayakawa Y, Zhan Y, et al (2004) Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. J Exp Med 199:879
Takeda K, Hayakawa Y, Smyth MJ, et al (2001) Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 7:94
Takeda K, Smyth MJ, Cretney E, et al (2002) Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 195:161
Takei F, McQueen KL, Maeda M, et al (2001) Ly49 and CD94/NKG2: developmentally regulated expression and evolution. Immunol Rev 181:90
Trapani JA, Smyth MJ (2002) Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2:735
Trinchieri G (1998) Immunobiology of interleukin-12. Immunol Res 17:269
Van den Broek MF, Kagi D, Zinkernagel RM, et al (1995) Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol 25:3514
Waldmann TA, Tagaya Y (1999) The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 17:19
Whiteside TL, Herberman RB (1995) The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol 7:704
Whiteside TL, Vujanovic NL and Herberman RB (1998) Natural killer cells and tumor therapy. Curr Top Microbiol Immunol 230:221
Williams NS, Moore TA, Schatzle JD, et al (1997) Generation of lytic natural killer 1.1+, Ly-49- cells from multipotential murine bone marrow progenitors in a stroma-free culture: definition of cytokine requirements and developmental intermediates. J Exp Med 186:1609
Wilson JL, Heffler LC, Charo J, et al (1999) Targeting of human dendritic cells by autologous NK cells. J Immunol 163:6365
Wilson MJ, Torkar M, Haude A, et al (2000) Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA 97:4778
Wooldridge JE, Weiner GJ (2003) CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response. Curr Opin Oncol 15:440
Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90:127
Yasumura S, Lin WC, Hirabayashi H, et al (1994) Immunotherapy of liver metastases of human gastric carcinoma with interleukin 2-activated natural killer cells. Cancer Res 54:3808
Yokoyama WM, Daniels BF, Seaman WE, et al (1995) A family of murine NK cell receptors specific for target cell MHC class I molecules. Semin Immunol 7:89
Yokoyama WM, Plougastel BF (2003) Immune functions encoded by the natural killer gene complex. Nat Rev Immunol 3:304
Yuan D, Koh CY, Wilder JA (1994) Interactions between B lymphocytes and NK cells. FASEB J, 8:1012
Zitvogel L (2002) Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med 195:F9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallace, M.E., Smyth, M.J. The role of natural killer cells in tumor control—effectors and regulators of adaptive immunity. Springer Semin Immun 27, 49–64 (2005). https://doi.org/10.1007/s00281-004-0195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-004-0195-x