Abstract
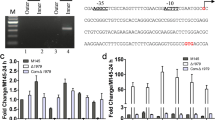
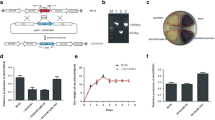
SCO3900, a putative target gene of the developmental regulator WhiI of Streptomyces coelicolor, encodes a protein similar to PadR from Pediococcus pentosaceus. The SCO3900 disruption mutant exhibited a bald phenotype with sparse aerial hyphae. However, a single copy of SCO3900 under the control of its native promoter could not complement the mutant phenotype. A fragment containing SCO3900 and its downstream three genes completely restored the mutant phenotype to the wild-type. These suggested that the disruption of SCO3900 exerted a polar effect on its downstream genes. The co-transcription of SCO3899, SCO3898, and SCO3897 with SCO3900 was confirmed by the RT–PCR. Moreover, overexpression of SCO3900 in the wild-type strain caused the same phenotype as that of the disruption mutant. The results suggested that SCO3900 encodes a negative regulator participating in morphological differentiation of S. coelicolor by influencing the expression of its downstream genes.




Similar content being viewed by others
References
Ainsa JA, Parry HD, Chater KF (1999) A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol Microbiol 34:607–619
Barthelmebs L, Lecomte B, Divies C et al (2000) Inducible metabolism of phenolic acids in Pediococcus pentosaceus is encoded by an autoregulated operon which involves a new class of negative transcriptional regulator. J Bacteriol 182:6724–6731
Chater KF (2001) Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr Opin Microbiol 4:667–673
Donahue TF, Henry SA (1981) myo-Inositol-1-phosphate synthase. Characteristics of the enzyme and identification of its structural gene in yeast. J Biol Chem 256:7077–7085
Fahey RC (2001) Novel thiols of prokaryotes. Annu Rev Microbiol 55:333–356
Gury J, Barthelmebs L, Tran NP et al (2004) Cloning, deletion, and characterization of PadR, the transcriptional repressor of the phenolic acid decarboxylase-encoding padA gene of Lactobacillus plantarum. Appl Environ Microbiol 70:2146–2153
Huillet E, Velge P, Vallaeys T et al (2006) LadR, a new PadR-related transcriptional regulator from Listeria monocytogenes, negatively regulates the expression of the multidrug efflux pump MdrL. FEMS Microbiol Lett 254:87–94
Kieser T, Bibb MJ, Buttner MJ et al (2000) Practical Streptomyces genetics. The John Innes Foundation, Norwich, UK
Madoori PK, Agustiandari H, Driessen AJ et al (2009) Structure of the transcriptional regulator LmrR and its mechanism of multidrug recognition. EMBO J 28:156–166
Pao SS, Paulsen IT, Saier MH Jr (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62:1–34
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Stangegaard M, Dufva IH, Dufva M (2006) Reverse transcription using random pentadecamer primers increases yield and quality of resulting cDNA. Biotechniques 40:649–657
Summers AO (2009) Damage control: regulating defenses against toxic metals and metalloids. Curr Opin Microbiol 12:138–144
Tian Y, Fowler K, Findlay K et al (2007) An unusual response regulator influences sporulation at early and late stages in Streptomyces coelicolor. J Bacteriol 189:2873–2885
Tran NP, Gury J, Dartois V et al (2008) Phenolic acid-mediated regulation of the padC gene, encoding the phenolic acid decarboxylase of Bacillus subtilis. J Bacteriol 190:3213–3224
Xie Z, Li W, Tian Y et al (2007) Identification and characterization of sawC, a whiA-like gene, essential for sporulation in Streptomyces ansochromogenes. Arch Microbiol 188:575–582
Acknowledgments
We are grateful to Professor Keith Chater (John Innes Centre, Norwich, UK) for providing E. coli ET12567/pUZ8002, pKC1132 and pSET152. We also thank Dr. Brenda Leskiw (University of Alberta, Canada) for the gift of apramycin. This work was supported by grants from the National Natural Science Foundation of China [30670033 to H. T.] and the Ministry of Science and Technology of China [2009CB118905 to H. T.].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, G., Tian, Y., Hu, K. et al. SCO3900, Co-Transcripted with Three Downstream Genes, Is Involved in the Differentiation of Streptomyces coelicolor . Curr Microbiol 60, 268–273 (2010). https://doi.org/10.1007/s00284-009-9536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9536-2