Abstract
Objective
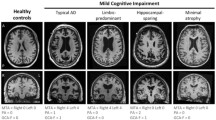
To assess the relationship between MRI-derived changes in whole-brain and ventricular volume with change in cognitive scores in Alzheimer’s disease (AD), mild cognitive impairment (MCI) and control subjects.
Material and methods
In total 131 control, 231 MCI and 99 AD subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort with T1-weighted volumetric MRIs from baseline and 12-month follow-up were used to derive volume changes. Mini mental state examination (MMSE), Alzheimer’s disease assessment scale (ADAS)-cog and trails test changes were calculated over the same period.
Results
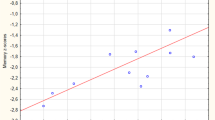
Brain atrophy rates and ventricular enlargement differed between subject groups (p < 0.0005) and in MCI and AD were associated with MMSE changes. Both measures were additionally associated with ADAS-cog and trails-B in MCI patients, and ventricular expansion was associated with ADAS-cog in AD patients. Brain atrophy (p < 0.0005) and ventricular expansion rates (p = 0.001) were higher in MCI subjects who progressed to AD within 12 months of follow-up compared with MCI subjects who remained stable. MCI subjects who progressed to AD within 12 months had similar atrophy rates to AD subjects.
Conclusion
Whole-brain atrophy rates and ventricular enlargement differed between patient groups and healthy controls, and tracked disease progression and psychological decline, demonstrating their relevance as biomarkers.


Similar content being viewed by others
References
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn (DSM-IV). American Psychological Association, Washington
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Jack CR Jr, Weigand SD, Shiung MM, Przybelski SA, O’Brien PC, Gunter JL, Knopman DS, Boeve BF, Smith GE, Petersen RC (2008) Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology 70:1740–1752
Freeborough PA, Fox NC (1997) The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imag 16:623–629
Fox NC, Freeborough PA, Rossor MN (1996) Visualisation and quantification of rates of atrophy in Alzheimer’s disease. Lancet 348:94–97
Frost C, Kenward MG, Fox NC (2004) The analysis of repeated ‘direct’ measures of change illustrated with an application in longitudinal imaging. Stat Med 23:3275–3286
O’Brien JT, Paling S, Barber R, Williams ED, Ballard C, McKeith IG, Gholkar A, Crum WR, Rossor MN, Fox NC (2001) Progressive brain atrophy on serial MRI in dementia with Lewy bodies, AD, and vascular dementia. Neurology 56:1386–1388
Jack CR Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC (2004) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62:591–600
Jack CR Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC (2005) Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65:1227–1231
Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H (2008) Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology 248:590–598
Fox NC, Schott JM (2004) Imaging cerebral atrophy: normal ageing to Alzheimer’s disease. Lancet 363:392–394
Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC (2003) A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60:989–994
Fox NC, Scahill RI, Crum WR, Rossor MN (1999) Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 52:1687–1689
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289:2819–2826
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, Whitwell L, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW (2008) The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 27:685–691
Jovicich J, Czanner S, Greve D, Haley E, van der KA, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A (2006) Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30:436–443
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imag 17:87–97
Narayana PA, Brey WW, Kulkarni MV, Sievenpiper CL (1988) Compensation for surface coil sensitivity variation in magnetic resonance imaging. Magn Reson Imaging 6:271–274
Freeborough PA, Fox NC, Kitney RI (1997) Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed 53:15–25
Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ (1999) Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imag 18:712–721
Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998) Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22:139–152
Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995) A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage 2:89–101
Lewis EB, Fox NC (2004) Correction of differential intensity inhomogeneity in longitudinal MR images. Neuroimage 23:75–83
Folstein MF, Folstein SE, Mchugh PR (1975) Mini-mental state—practical method for grading cognitive state of patients for clinician. J Psychiatr Res 12:189–198
Rosen WG, Mohs RC, Davis K (1984) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141:1356–1364
Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: therapy and clinical interpretation. AZ Neuropsychological, Tucson
Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN (2000) Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects [see comments]. Arch Neurol 57:339–344
Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, Fox NC, Rossor MN (2006) Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol 60:145–147
Wang D, Chalk JB, Rose SE, de Zubicaray G, Cowin G, Galloway GJ, Barnes D, Spooner D, Doddrell DM, Semple J (2002) MR image-based measurement of rates of change in volumes of brain structures. Part II: application to a study of Alzheimer’s disease and normal aging. Magn Reson Imaging 20:41–48
Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC (2002) Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci USA 99:4703–4707
Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, Rossor MN, Fox NC (2003) Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: longitudinal MRI study. Lancet 362:1121–1122
Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC (2005) Longitudinal volumetric MRI change and rate of cognitive decline. Neurology 65:565–571
Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA (2003) Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 61:487–492
Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC (2005) Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology 65:119–124
Kaye JA, Moore MM, Dame A, Quinn J, Camicioli R, Howieson D, Corbridge E, Care B, Nesbit G, Sexton G (2005) Asynchronous regional brain volume losses in presymptomatic to moderate AD. J Alzheimers Dis 8:51–56
Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, Whitwell JL, Jenkins L, Black RS, Grundman M, Fox NC (2008) Volumetric MRI and cognitive measures in Alzheimer disease: comparison of markers of progression. J Neurol 255:567–574
Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R (2008) Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain 131:2443–2454
Lehericy S, Marjanska M, Mesrob L, Sarazin M, Kinkingnehun S (2007) Magnetic resonance imaging of Alzheimer’s disease. Eur Radiol 17:347–362
Acknowledgements
This work was undertaken in University College London Hospitals/University College London, which received a proportion of funding from the UK Department of Health’s National Institute of Health Research Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer’s Research Trust Co-ordinating Centre. Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative database (www.loni.ucla.edu/ADNI). A list of authors that contributed to varied aspects of design and implementation of the ADNI study, but did not contribute to the writing of this article can be found at http://www.loni.ucla.edu/ADNI/About/About_InvestigatorsTable.shtml. ADNI is funded by the National Institute of Ageing, the National Institute of Biomedical Imaging and Bioengineering (NBIB), and the Foundation of the National Institutes of Health, contributions from the following companies and organisations: Pfitzer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, the Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Ageing (ISOA), with participation from the U.S. Food and Drug Administration. N Fox is supported by the Medical Research Council (UK) Senior Clinical Fellowship. J Barnes is supported by Alzheimer’s Research Trust (UK) Research Fellowship with the kind support of the Kirby Laing Foundation. K Leung was funded by a Technology Strategy Board Grant. The authors would like to thank Chris Frost for his statistical advice. Author contributions were as follows: Drafting and editing: MCE, JB, NCF, LGK; quality control of MRI images was carried out by SLC, MCE and MB; data download performed and MR data issues dealt with by CN, RB and KL; AD and RB were responsible for developing the propagation technique used; MCE conducted the statistical analysis under supervision of LGK.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, M.C., Barnes, J., Nielsen, C. et al. Volume changes in Alzheimer’s disease and mild cognitive impairment: cognitive associations. Eur Radiol 20, 674–682 (2010). https://doi.org/10.1007/s00330-009-1581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1581-5