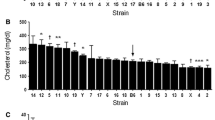
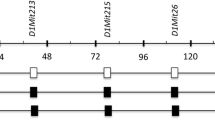
Abstract
Obesity is associated with increased susceptibility to dyslipidemia, insulin resistance, and hypertension, a combination of traits that comprise the traditional definition of the metabolic syndrome. Recent evidence suggests that obesity is also associated with the development of nonalcoholic fatty liver disease (NAFLD). Despite the high prevalence of obesity and its related conditions, their etiologies and pathophysiology remains unknown. Both genetic and environmental factors contribute to the development of obesity and NAFLD. Previous genetic analysis of high-fat, diet-induced obesity in C57BL/6J (B6) and A/J male mice using a panel of B6-ChrA/J/NaJ chromosome substitution strains (CSSs) demonstrated that 17 CSSs conferred resistance to high-fat, diet-induced obesity. One of these CSS strains, CSS-17, which is homosomic for A/J-derived chromosome 17, was analyzed further and found to be resistant to diet-induced steatosis. In the current study we generated seven congenic strains derived from CCS-17, fed them either a high-fat, simple-carbohydrate (HFSC) or low-fat, simple-carbohydrate (LFSC) diet for 16 weeks and then analyzed body weight and related traits. From this study we identified several quantitative trait loci (QTLs). On a HFSC diet, Obrq13 protects against diet-induced obesity, steatosis, and elevated fasting insulin and glucose levels. On the LFSC diet, Obrq13 confers lower hepatic triglycerides, suggesting that this QTL regulates liver triglycerides regardless of diet. Obrq15 protects against diet-induced obesity and steatosis on the HFSC diet, and Obrq14 confers increased final body weight and results in steatosis and insulin resistance on the HFSC diet. In addition, on the LFSC diet, Obrq 16 confers decreased hepatic triglycerides and Obrq17 confers lower plasma triglycerides on the LFSC diet. These congenic strains provide mouse models to identify genes and metabolic pathways that are involved in the development of NAFLD and aspects of diet-induced metabolic syndrome.







Similar content being viewed by others
References
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553
Angulo P (2002) Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231
Balkau B, Charles MA (1999) Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 16:442–443
Bell CG, Walley AJ, Froguel P (2005) The genetics of human obesity. Nat Rev Genet 6:221–234
Black BL, Croom J, Eisen EJ, Petro AE, Edwards CL et al (1998) Differential effects of fat and sucrose on body composition in A/J and C57BL/6 mice. Metabolism 47:1354–1359
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD et al (2004) Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40:1387–1395
Buchner DA, Burrage LC, Hill AE, Yazbek SN, O’Brien WE et al (2008) Resistance to diet-induced obesity in mice with a single substituted chromosome. Physiol Genomics 35:116–122
Bultman SJ, Michaud EJ, Woychik RP (1992) Molecular characterization of the mouse agouti locus. Cell 71:1195–1204
Butler AA, Cone RD (2001) Knockout models resulting in the development of obesity. Trends Genet 17:S50–S54
Clark JM, Diehl AM (2003) Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 289:3000–3004
Colinayo VV, Qiao JH, Wang X, Krass KL, Schadt E et al (2003) Genetic loci for diet-induced atherosclerotic lesions and plasma lipids in mice. Mamm Genome 14:464–471
Collins S, Daniel KW, Petro AE, Surwit RS (1997) Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 138:405–413
Collins S, Martin TL, Surwit RS, Robidoux J (2004) Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81:243–248
Diehl AM, Goodman Z, Ishak KG (1988) Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology 95:1056–1062
Ellacott KL, Murphy JG, Marks DL, Cone RD (2007) Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 148:6186–6194
Fan CY, Pan J, Chu R, Lee D, Kluckman KD et al (1996) Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem 271:24698–24710
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Ground KE (1982) Liver pathology in aircrew. Aviat Space Environ Med 53:14–18
Hall P de la M, Kirsch R (2004) Pathology of hepatic steatosis, NASH and related conditions. In: Farrell GC, George J, Hall P de la M, McCullough AJ (eds), Fatty liver disease NASH and related disorders. Wiley-Blackwell, New York, chap 2
Haluzik MM, Lacinova Z, Dolinkova M, Haluzikova D, Housa D et al (2006) Improvement of insulin sensitivity after peroxisome proliferator-activated receptor-alpha agonist treatment is accompanied by paradoxical increase of circulating resistin levels. Endocrinology 147:4517–4524
Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK et al (2000) Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem 275:28918–28928
Hilden M, Christoffersen P, Juhl E, Dalgaard JB (1977) Liver histology in a ‘normal’ population—examinations of 503 consecutive fatal traffic casualties. Scand J Gastroenterol 12:593–597
Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q et al (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141
Ishimori N, Li R, Kelmenson PM, Korstanje R, Walsh KA et al (2004a) Quantitative trait loci analysis for plasma HDL-cholesterol concentrations and atherosclerosis susceptibility between inbred mouse strains C57BL/6J and 129S1/SvImJ. Arterioscler Thromb Vasc Biol 24:161–166
Ishimori N, Li R, Kelmenson PM, Korstanje R, Walsh KA et al (2004b) Quantitative trait loci that determine plasma lipids and obesity in C57BL/6J and 129S1/SvImJ inbred mice. J Lipid Res 45:1624–1632
Khashab MA, Liangpunsakul S, Chalasani N (2008) Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr Gastroenterol Rep 10:73–80
Kim JA, Wei Y, Sowers JR (2008) Role of mitochondrial dysfunction in insulin resistance. Circ Res 102:401–414
Lango H, Weedon MN (2008) What will whole genome searches for susceptibility genes for common complex disease offer to clinical practice? J Intern Med 263:16–27
Lee RG (1989) Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol 20:594–598
Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR (2009) Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int 29:113–119
Lu D, Willard D, Patel IR, Kadwell S, Overton L et al (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371:799–802
Ludwig J, Viggiano TR, McGill DB, Oh BJ (1980) Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55:434–438
Matin A, Collin GB, Asada Y, Varnum D, Nadeau JH (1999) Susceptibility to testicular germ-cell tumours in a 129.MOLF-Chr 19 chromosome substitution strain. Nat Genet 23:237–240
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC et al (1999) Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116:1413–1419
McCullough AJ (2004) NAFLD/NASH is not just a ‘Western’ problem: some perspectives on NAFLD/NASH from the East. In: Farrell GC, George J, Hall P de la M, McCullough AJ (eds), Fatty liver disease: NASH and related disorders. Wiley-Blackwell, New York, chap 18
Michaud EJ, Bultman SJ, Stubbs LJ, Woychik RP (1993) The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes Dev 7:1203–1213
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Mills E, Kuhn CM, Feinglos MN, Surwit R (1993) Hypertension in CB57BL/6 J mouse model of non-insulin-dependent diabetes mellitus. Am J Physiol 264:R73–R78
Nadeau JH, Singer JB, Matin A, Lander ES (2000) Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24:221–225
Nathan BM, Hodges CA, Palmert MR (2006a) The use of mouse chromosome substitution strains to investigate the genetic regulation of pubertal timing. Mol Cell Endocrinol 254–255:103–108
Nathan BM, Hodges CA, Supelak PJ, Burrage LC, Nadeau JH et al (2006b) A quantitative trait locus on chromosome 6 regulates the onset of puberty in mice. Endocrinology 147:5132–5138
Noguchi T, Noguchi M (1985) A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst 75:385–392
Ong KK, Dunger DB (2001) Developmental aspects in the pathogenesis of type 2 diabetes. Mol Cell Endocrinol 185:145–149
Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ et al (2005) The human obesity gene map: the 2004 update. Obes Res 13:381–490
Peterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE et al (2007) Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet 39:1483–1487
Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G et al (2006) The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14:529–644
Reaven GM (1988) Banting lecture 1998. Role of insulin resistance in human disease. Diabetes 37:1595–1607
Salmon DM, Flatt JP (1985) Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int J Obes 9:443–449
Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J et al (2001) G-protein signaling through tubby proteins. Science 292:2041–2050
Shao H, Burrage LC, Sinasac DS, Hill AE, Ernest SR et al (2008) Genetic architecture of complex traits: Large phenotypic effects and pervasive epistasis. Proc Natl Acad Sci 50:19910–19914
Singer JB, Hill AE, Burrage LC, Olszens KR, Song J et al (2004) Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304:445–448
Stunkard AJ (1996) Current views on obesity. Am J Med 100:230–236
Stunkard AJ, Foch TT, Hrubec Z (1986) A twin study of human obesity. JAMA 256:51–54
Stylianou IM, Tsaih SW, DiPetrillo K, Ishimori N, Li R et al (2006) Complex genetic architecture revealed by analysis of high-density lipoprotein cholesterol in chromosome substitution strains and F2 crosses. Genetics 174:999–1007
Suriawinata A, Fiel MI (2004) Liver pathology in obesity. Semin Liver Dis 24:363–370
Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN (1988) Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167
Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE et al (1995) Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44:645–651
Taylor BA, Phillips SJ (1997) Obesity QTLs on mouse chromosomes 2 and 17. Genomics 43:249–257
Tomoda H, Omura S (2007) Potential therapeutics for obesity and atherosclerosis: inhibitors of neutral lipid metabolism from microorganisms. Pharmacol Ther 115:375–389
Weiser M, Frishman WH, Michaelson MD, Abdeen MA (1997) The pharmacologic approach to the treatment of obesity. J Clin Pharmacol 37:453–473
Xia Z, Sniderman AD, Cianflone K (2002) Acylation-stimulating protein (ASP) deficiency induces obesity resistance and increased energy expenditure in ob/ob mice. J Biol Chem 277:45874–45879
Youngren KK, Nadeau JH, Matin A (2003) Testicular cancer susceptibility in the 129.MOLF-Chr19 mouse strain: additive effects, gene interactions and epigenetic modifications. Hum Mol Genet 12:389–398
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341
Acknowledgments
This work was supported by NIH grants DK075040 (CMC) and RR12305 (JHN), NIH Metabolism training grant T32-DK007319 (CAM), NIH training grant GM08613 (LCB), and NIH grant T32-GM07250 to the CASE MSTP (LCB).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. A. Millward and L. C. Burrage contributed equally to this work.
Electronic supplementary material
Supplementary Figure 1. Food intake on HFSC diet.
Food intakes were measured weekly during the HFSC diet study. The values are the mean ± SEM for n = 8-22 mice per group and are represented as grams per mouse per day. (*P < 0.005 compared to B6, Bonferonni’s correction for multiple testing). (abbreviations: B6 for C57BL/6J, A17 for CSS-17, C1 for 17C1, C2 for 17C2, C3 for 17C3, C4 for 17C4, C5 for 17C5, C6 for 17C6 and C7 for 17C7). (TIFF 13873 kb)
Supplementary Table 1. Markers used for congenic panel of CSS-17.
Seven congenic strains were generated. All primer sequences for micorsatellite markers were obtained from the Mouse Genome Informatics website http://www.informatics.jax.org/. (TIFF 12497 kb)
Supplementary Table 2 Metabolic analysis of CSS-17 and congenic panel on high fat diet.
Mice were fed a HFSC diet for 16 weeks and fasted overnight. The values are the mean±SEM for each strain tested (n = 8-22 mice per group). (aP < 0.005 for 17C1 compared to B6, bP < 0.005 for 17C5 compared to 17C6, cP < 0.005 for 16C6 to 17C7 with Bonferonni’s correction for multiple testing) (Abbreviations are as follows TG, triglycerides; FFA, free fatty acids; CHOL, cholesterol). (TIFF 27508 kb)
Supplementary Table 3. Metabolic analysis of CSS-17 and congenic panel on LFSC diet.
Mice were fed a LFSC diet for 16 weeks and fasted overnight. The values are the mean±SEM for each strain tested (n = 8-15 mice per group). (aP < 0.005 for 17C1 compared to B6, bP < 0.005 for 17C2 compared to 17C1, cP < 0.005 for 16C4 to 17C3 with Bonferonni’s correction for multiple testing) (TIFF 17245 kb)
Rights and permissions
About this article
Cite this article
Millward, C.A., Burrage, L.C., Shao, H. et al. Genetic factors for resistance to diet-induced obesity and associated metabolic traits on mouse chromosome 17. Mamm Genome 20, 71–82 (2009). https://doi.org/10.1007/s00335-008-9165-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-008-9165-2