Abstract
Introduction
The pathophysiology involved in human neonatal high-pressure hydrocephalus (HC) includes both cerebrospinal fluid (CSF) malabsorption and obstruction.
Objective
The aim was to estimate the relative contribution between CSF malabsorption and obstruction in three different etiological groups of neonatal high-pressure HC by assessment of specific CSF biomarkers indicative of growth factor- and fibrosis-related CSF malabsorption (transforming growth factor beta-1 (TGF beta-1), aminoterminal propeptide of type 1 collagen (PC1NP)].
Materials and methods
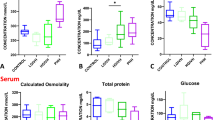
Patients were subdivided into three groups. Group A: spina bifida HC (n=12); group B: non-haemorrhagic triventricular HC (n=4); and group C: posthaemorrhagic HC (n=6). To exclude for confounding differences in pro-inflammatory state between the three groups, interleukin-6 (IL-6) CSF concentrations were assessed. Consecutively, the CSF concentrations of TGF beta-1 and PC1NP were compared between the different groups.
Results
Median CSF concentrations of IL-6 were low and did not differ between groups. Median CSF concentrations of PC1NP were significantly lower in group A (median: 180 ng/ml, range 90–808) than in group C (median: 1,060, range 396–1194; p=0.002). TGF beta-1 concentrations were significantly higher in group C (median 355 pg/ml, range 129–843) than in groups A (median 103, range 78–675 pg/ml) and B (median 120 pg/ml, range 91–188; p=0.01 and 0.03, respectively).
Conclusions
In neonatal posthaemorrhagic HC, high concentrations of malabsorption-related biomarkers contrast with lower concentrations in SB and non-haemorrhagic triventricular HC. During the early development of high pressure HC in SB neonates, CSF biomarkers strongly indicate that CSF obstruction contributes more to the development of HC than malabsorption.


Similar content being viewed by others
References
Mori K (1995) Current concept of hydrocephalus: evolution of new classifications. Childs Nerv Syst 11:523–532
Bell WO (1995) Cerebrospinal fluid reabsorption. Pediatr Neurosurg 23:42–53
Friede RL (1989) Developmental neuropathology. Springer, Berlin Heidelberg New York, pp 220–230
Pattisapu JV, Morgan FW, Trumble ER, Gegg CA (2003) Hydrocephalus-time for a unified theory or a multi-directional approach? Eur J Surg 13:S55
Koch D, Wagner W (2004) Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome? Childs Nerv Syst 20:405–411
Li X, Miyajima M, Arai H (2005) Analysis of TGF-beta 2 and TGF-beta 3 expression in the hydrocephalic H-Tx rat brain. Childs Nerv Syst 21:32–38
Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342:1350–1358
Johnson MD, Gold LI, Moses HL (1992) Evidence for transforming growth factor β expression in human leptomeningeal cells and transforming growth factor β-like activity in human cerebrospinal fluid. Lab Invest 67:360–368
Bottner M, Krieglstein K, Unsicker K (2000) The transforming growth factor-betas: structure, signaling and roles in nervous system development and functions. J Neurochem 75:2227–2240
Norenberg MD (1994) Astrocyte responses to CNS injury. J Neuropathol Exp Neurol 53:213–220
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefiled LM, Heine UI, Liotta LA, Falanga V, Kehrl JH (1986) Transforming growth factor type β rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A 82:4167–4171
Heep A, Stoffel-Wagner B, Bartmann P, Benseler S, Schaller C, Groneck P, Obladen M, Felderhoff-Mueser U (2004) Vascular endothelial growth factor (VEGF) and transforming growth factor beta-1 (TGF beta-1) are highly expressed in the cerebrospinal fluid (CSF) of premature infants with posthemorrhagic hydrocephalus Pediatr Res 56:768–774
Flood C, Akinwunmi J, Lagord C, Daniel M, Berry M, Jackowski A, Logan A (2001) Transforming growth factor-beta1 in the cerebrospinal fluid of patients with subarachnoid hemorrhage: titers derived from exogeneous and endogeneous sources. J Cereb Blood Flow Metab 21:157–162
Morganti-Kossmann MC, Hans V, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T (1999) TGF-beta 1 is elevated in the CSF of patients with severe brain injuries and parallels blood-brain-barrier function. J Neurotrauma 16:617–628
Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T (1999) IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood–brain barrier function. J Neuroimmunol 101:211–221
Kitisawa K, Tada T (1994) Elevation of transforming growth factor β-1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 25:1400–1404
Wang JF, Olson ME, Ma L, Brigstock DR, Hart DA (2004) Connective tissue growth factor siRNA modulates mRNA levels for a subset of molecules in normal and TGF-beta 1 stimulated porcine skin fibroblasts. Wound Repair Regen 12:205–216
Schwab JM, Beschorner R, Nguyen TD, Meyermann R, Schluesener HJ (2001) Differential cellular accumulation of connective tissue growth factor defines a subset of reactive astrocytes, invading fibroblasts, and endothelial cells following central nervous system injury in rats and humans. J Neurotrauma 18:377–388
Schwab JM, Postler E, Nguyen TD, Mittelbronn M, Meyermann R, Schluesener HJ (2000) Connective tissue growth factor is expressed by a subset of reactive astrocytes in human cerebral infarction. Neuropathol Appl Neurobiol 26:434–440
Tada T, Kanaji M, Kobayashi S (1994) Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. J Neuroimmunol 50:153–158
Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG (1999) Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol 277:263–271
Nakazato F, Tada T, Sekiguchi Y, Murakami K, Yanagisawa S, Tanaka Y, Hongo K (2002) Disturbed spatial learning of rats after intraventricular administration of transforming growth factor-beta 1. Neurol Med Chir 42:151–156
Moinuddin SM, Tada T (2000) Study of cerebrospinal fluid flow dynamics in TGFβ1 induced chronic hydrocephalic mice. Neurol Res 22:215–222
Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L (1995) Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol 147:53–67
Cai X, Pattisapu JV, Tarnuzzer RW, Fernandez-Valle C, Gibson JS (1999) TGF-beta 1 expression is reduced in hydrocephalic H-Tx rat brain. Eur J Pediatr Surg 51(Suppl 1):35–38
Sajanti J, Heikkinen E, Majamaa K (2000) Transient increase in procollagen propeptides in the CSF after subarachnoid hemorrhage. Neurology 55:359–363
Massicotte EM, Del Bigio MR (1999) Human villi response to subarachnoideal hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg 91:80–84
Motohashi O, Suzuki M, Shida N (1995) Subarachnoid hemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunohistochemical study. Acta Neurochir 136:88–91
Kim RC, Talbert WM, Choe W, Choi BH (1989) Massive craniospinal collagen deposition after persistent postoperative intraventricular bleeding. Neurosurgery 24:771–775
Suzuki S, Ishii M, Ottomo M (1977) Changes in the subarachnoid space after subarachnoidal haemorrhage in the dog: scanning electron microscopic observation. Acta Neurochir 39:1–14
Heep A, Stoffel-Wagner B, Soditt V, Aring C, Groneck P, Bartmann P (2002) Procollagen I C-propeptide in the cerebrospinal fluid of neonates with posthemorrhagic hydrocephalus. Arch Dis Child Fetal Neonatal Ed 87:F34–F36
Heep A, Stoffel-Wagner B, Bartmann P (2000) Procollagen propeptides in the cerebrospinal fluid of neonates with posthemorrhagic hydrocephalus (PHHC). Biol Neonate 78:153
Sajanti J, Björkstrand AS, Finnilä S, Heikkinen E, Peltonen J, Majamaa K (1999) Increase of collagen synthesis and deposition in the arachnoid and the dura following subarachnoid hemorrhage in the rat. Biochim Biophys Acta 1454:209–216
Sajanti J, Majamaa K (1999) Detection of meningeal fibrosis after subarachnoid hemorrhage by assaying procollagen propeptides in cerebrospinal fluid. J Neurol Neurosurg Psychiatry 67:185–188
Risteli L, Risteli J (1987) Analysis of extracellular matrix proteins in biological fluids. Methods Enzymol 145:391–411
Risteli J, Risteli L (1995) Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J Hepatol 22:77–81
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real time ultrasound. Arch Dis Child 56:900–904
Volpe JJ (2001) Neurology of the newborn. WB Saunders, Philadelphia, pp 428–493
Heep A, Engelskirchen R, Holschneider A, Groneck P (2001) Primary intervention for posthemorrhagic hydrocephalus in very low birthweight infants by ventriculostomy. Childs Nerv Syst 17:47–51
Paneth N (1999) Classifying brain damage in preterm infants. J Pediatr 134:527–529
Xiao L, Li XQ, Zhang HX (2002) Effects of nao-yi-an granule on the intercellular expression of IL-6 in the experimental intracerebral hemorrhagic brain of rats. Hunan Yi Ke Da Xue Xue Bao 28:13–16
Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura KI (2001) Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res 23:724–730
Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Whalen MJ, DeKosky ST (1997) Comparison of the interleukin-6 and interleukin-10 response in children after severe traumatic brain injury or septic shock. Acta Neurochir 70:96–97
Heep A, Schaller C, Rittmann N, Himbert U, Marklein G, Bartmann P (2004) Multiple brain abscesses in an extremely preterm infant: treatment surveillance with interleukin-6 in the CSF. Eur J Pediatr 163:44–45
Baumeister FA, Pohl-Koppe A, Hofer M, Kim JO, Weiss M (2000) IL-6 in CSF during ventriculitis in preterm infants with posthemorrhagic hydrocephalus. Infection 28:234–236
Del Bigio MR, Zhang YW (1998) Cell death, axonal damage and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol 154:157–169
Johanson CE, Jones HC (2001) Promising vistas in hydrocephalus and cerebrospinal fluid research. Trends Neurosci 24:631–632
Boillat CA, Jones HC, Kaiser GL, Harris NG (1997) Ultrastructural changes in the deep cortical pyramid cells of infant rats with inherited hydrocephalus and the effect of shunt treatment. Exp Neurol 147:377–388
Xia XY, Ikeda T, Xia XY, Ikenoue T (2000) Differential neurotrophin levels in cerebrospinal fluid and their changes during development in newborn rat. Neurosci Lett 280:220–222
Takei F, Sato O (1995) Morphological analysis of progressive hydrocephalus and shunt-dependent arrested hydrocephalus. An experimental study. Pediatr Neurosurg 23:246–253
Acknowledgements
We gratefully acknowledge the excellent technical assistance by Martina Schmidt and Anne Thiel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heep, A., Bartmann, P., Stoffel-Wagner, B. et al. Cerebrospinal fluid obstruction and malabsorption in human neonatal hydrocephaly. Childs Nerv Syst 22, 1249–1255 (2006). https://doi.org/10.1007/s00381-006-0102-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0102-y