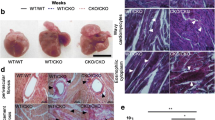
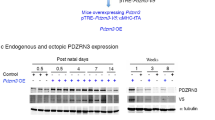
Abstract
Beta-catenin is a component of the intercalated disc in cardiomyocytes, but can also be involved in signalling and activation of gene transcription. We wanted to determine how long-term changes in beta-catenin expression levels would affect mature cardiomyocytes. Conditional transgenic mice that either lacked beta-catenin or that expressed a non-degradable form of beta-catenin in the adult ventricle were created. While mice lacking beta-catenin in the ventricle do not have an overt phenotype, mice expressing a non-degradable form develop dilated cardiomyopathy and do not survive beyond 5 months. A detailed analysis could reveal that this phenotype is correlated with a distinct localisation of beta-catenin in adult cardiomyocytes, which cannot be detected in the nucleus, no matter how much protein is present. Our report is the first study that addresses long-term effects of either the absence of beta-catenin or its stabilisation on ventricular cardiomyocytes and it suggests that beta-catenin’s role in the nucleus may be of little significance in the healthy adult heart.







Similar content being viewed by others
References
Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG (1997) Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80:88–94
Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW (2007) Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res 100:1353–1362
Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R (2001) Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264
Chen J, Kubalak SW, Chien KR (1998) Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development 125:1943–1949
Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, Kerkela R, Molkentin JD, Liao R, Salomon RN, Patten R, Force T (2006) The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol 26:4462–4473
Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95:2979–2984
Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE (2007) Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest 117:1794–1804
Ehler E, Horowits R, Zuppinger C, Price RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM, Perriard JC (2001) Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol 153:763–772
Fagotto F, Gluck U, Gumbiner BM (1998) Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol 8:181–190
Forbes MS, Sperelakis N (1985) Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell 17:605–648
Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J (2008) Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 103:485–492
Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ (2006) Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 116:2012–2021
Gehmlich K, Geier C, Milting H, Furst D, Ehler E (2008) Back to square one: what do we know about the functions of Muscle LIM Protein in the heart? J Muscle Res Cell Motil 29:155–158
Gottardi CJ, Gumbiner BM (2004) Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167:339–349
Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22:2308–2341
Gupta P, Bilinska ZT, Sylvius N, Boudreau E, Veinot JP, Labib S, Bolongo PM, Hamza A, Jackson T, Ploski R, Walski M, Grzybowski J, Walczak E, Religa G, Fidzianska A, Tesson F (2010) Genetic and ultrastructural studies in dilated cardiomyopathy patients: a large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res Cardiol 105:365–377
Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121:3529–3537
Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW, Koo BK, Chae IH, Shin CS, Oh BH, Choi YS, Park YB, Kim HS (2006) Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem 281:30979–30989
Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T (2003) Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA 100:4610–4615
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM (1999) Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18:5931–5942
Hirschy A, Schatzmann F, Ehler E, Perriard JC (2006) Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol 289:430–441
Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172–1183
Kapiloff MS, Jackson N, Airhart N (2001) mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci 114:3167–3176
Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111:943–955
Koike M, Kose S, Furuta M, Taniguchi N, Yokoya F, Yoneda Y, Imamoto N (2004) beta-Catenin shows an overlapping sequence requirement but distinct molecular interactions for its bidirectional passage through nuclear pores. J Biol Chem 279:34038–34047
Leu M, Ehler E, Perriard JC (2001) Characterisation of postnatal growth of the murine heart. Anat Embryol (Berl) 204:217–224
Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R (2002) Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell 3:171–181
Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM (2007) Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA 104:9313–9318
Lombardi R, Dong J, Rodriguez G, Bell A, Leung TK, Schwartz RJ, Willerson JT, Brugada R, Marian AJ (2009) Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res 104:1076–1084
Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100:3299–3304
Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, Salpingidou G, Wilson RG, Ellis JA, Hutchison CJ (2006) The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J 25:3275–3285
Mertens C, Kuhn C, Franke WW (1996) Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025
Moriyama A, Kii I, Sunabori T, Kurihara S, Takayama I, Shimazaki M, Tanabe H, Oginuma M, Fukayama M, Matsuzaki Y, Saga Y, Kudo A (2007) GFP transgenic mice reveal active canonical Wnt signal in neonatal brain and in adult liver and spleen. Genesis 45:90–100
Perez-Ruiz A, Ono Y, Gnocchi VF, Zammit PS (2008) beta-Catenin promotes self-renewal of skeletal-muscle satellite cells. J Cell Sci 121:1373–1382
Perriard JC, Hirschy A, Ehler E (2003) Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med 13:30–38
Perrot A, Hussein S, Ruppert V, Schmidt HH, Wehnert MS, Duong NT, Posch MG, Panek A, Dietz R, Kindermann I, Bohm M, Michalewska-Wludarczyk A, Richter A, Maisch B, Pankuweit S, Ozcelik C (2009) Identification of mutational hot spots in LMNA encoding lamin A/C in patients with familial dilated cardiomyopathy. Basic Res Cardiol 104:90–99
Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F (2007) Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol 43:319–326
Rhee D, Sanger JM, Sanger JW (1994) The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskelet 28:1–24
Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ (2007) A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol 178:897–904
Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS (2002) Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90:458–464
Sheikh F, Chen Y, Liang X, Hirschy A, Stenbit AE, Gu Y, Dalton ND, Yajima T, Lu Y, Knowlton KU, Peterson KL, Perriard JC, Chen J (2006) alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture. Circulation 114:1046–1055
Snabaitis AK, D’Mello R, Dashnyam S, Avkiran M (2006) A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem 281:20252–20262
Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B (2008) APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell 32:652–661
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456:980–984
Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP (2007) Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128:141–156
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250
Vincan E, Leet CS, Reyes NI, Dilley RJ, Thomas RJ, Phillips WA (2000) Sodium butyrate-induced differentiation of human LIM2537 colon cancer cells decreases GSK-3beta activity and increases levels of both membrane-bound and Apc/axin/GSK-3beta complex-associated pools of beta-catenin. Oncol Res 12:193–201
Wheeler MA, Warley A, Roberts RG, Ehler E, Ellis JA (2010) Identification of an emerin-beta-catenin complex in the heart important for intercalated disc architecture and beta-catenin localisation. Cell Mol Life Sci 67:781–796
Zelarayan L, Gehrke C, Bergmann MW (2007) Role of beta-catenin in adult cardiac remodeling. Cell Cycle 6:2120–2126
Zemljic-Harpf AE, Miller JC, Henderson SA, Wright AT, Manso AM, Elsherif L, Dalton ND, Thor AK, Perkins GA, McCulloch AD, Ross RS (2007) Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol 27:7522–7537
Zhou J, Qu J, Yi XP, Graber K, Huber L, Wang X, Gerdes AM, Li F (2007) Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 292:H270–H276
Acknowledgments
This work was supported by a grant from the Swiss National Science Foundation (grant#3100-063486.00), a grant from the Gebert Rüf Foundation (grant # P038-01), by the Swiss Cardiovascular Training and Research Network (SCARTNet) and the Swiss University Conference (SUK). Work in the laboratory of Dr Ehler is supported by a Career Establishment Grant by the MRC. We are grateful for many helpful discussions with all members of the Perriard group, especially Dr Jaya Krishnan. In addition we thank Dr Stephan Lange (UCSD, San Diego, USA) and Dr Matthew Wheeler (King’s College London, London, UK) for critically reading the manuscript. We thank Professors Rolf Kemler (Max-Planck Institute, Freiburg, Germany) and Ju Chen (UCSD, San Diego, USA) who provided us with beta-catenin floxed mice and MLC2v-Cre mice. We thank Dr Andrea Domenighetti (University Hospital, Lausanne, Switzerland) and Dr P. Jonsen (VisualSonics Inc., Toronto, Canada) for performing ECG studies on cKO and cΔex3 animals. The help of Dr Nicolas Lindegger (Department of Physiology, Bern, Switzerland) for the isolation of adult cardiomyocytes is also acknowledged. We are grateful to Dr Katja Gehmlich (University College London) for the donation of HL-1 cells.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2010_101_MOESM1_ESM.tif
Supplementary Figure 1: Beta-catenin protein only becomes fully absent in the ventricle after two months. Confocal micrographs of frozen sections stained with monoclonal mouse anti beta-catenin from WT (left column) and cKO (right column) mice at postnatal day 3 (P3), one month, two months, six months and fifteen months of age. The beta-catenin signal at P3 and one month is comparable between WT and cKO and only disappears at two months, indicating a slow turnover of the protein at the intercalated disc. Bar in A equals 10 micometres, bar in C equals 20 micrometres (TIFF 6.83 mb)
395_2010_101_MOESM2_ESM.tif
Supplementary Figure 2: Intercalated disc organisation is hardly affected by the absence of beta-catenin. Confocal micrographs of frozen sections of two months old WT (A, B, E, F, I, J) and cKO ventricles (C, D, G, H, K, L) immunostained for cadherins (A, C), beta-catenin (B, D, F, H, J, L), desmoplakin (E, G) and connexin-43 (I, K). Cadherin staining is slightly more pronounced in the cKO (compare C with A), however neither desmosomes nor gap junctions seem to be altered. Occasional residual beta-catenin containing intercalated discs indicate a lack of recombination (arrow in H). Bar equals 20 micrometres (TIFF 9 mb)
395_2010_101_MOESM3_ESM.tif
Supplementary Figure 3: Nuclear accumulation of beta-catenin following lithium chloride stimulation and inhibition of nuclear export by leptomycin B can be detected in HL-1 cells but not in NRC. Confocal micrographs of HL-1 cells (top two rows) and NRC (bottom two rows) stained 33 for beta-catenin and with DAPI to visualise nuclei. Cells were grown either in control medium, in medium supplemented with lithium chloride (LiCl), leptomycin B (LMB) or both. Bar equals 10 micrometres (TIFF 5.19 mb)
Rights and permissions
About this article
Cite this article
Hirschy, A., Croquelois, A., Perriard, E. et al. Stabilised beta-catenin in postnatal ventricular myocardium leads to dilated cardiomyopathy and premature death. Basic Res Cardiol 105, 597–608 (2010). https://doi.org/10.1007/s00395-010-0101-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-010-0101-8