Abstract
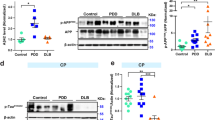
Alzheimer’s disease (AD), Pick’s disease (PiD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and dementia with Lewy bodies (DLB) are diseases associated with the accumulation of tau or α-synuclein. In AD, β-amyloid (Aβ)-associated caspase activation and cleavage of tau at Asp421 (ΔTau) may be an early step in neurofibrillary tangle (NFT) formation. To examine whether ΔTau accumulates in other diseases not characterized by extracellular Aβ accumulation, we examined PiD, PSP, and CBD cases in comparison to those without extensive tau accumulation including frontotemporal lobar degeneration without Pick bodies (FTLD) and control cases. Additionally, we studied ΔTau accumulation in DLB cases associated with intracellular α-synuclein. ΔTau was observed in all disease cases except non-PiD FTLD and controls. These results demonstrate that the accumulation of ΔTau may represent a common pathway associated with abnormal accumulation of intracellular tau or α-synuclein and may be relatively less dependent on the extracellular accumulation of Aβ in non-AD dementias.





Similar content being viewed by others
References
Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, Iritani S, Tsuchiya K, Iseki E, Yagishita S, Oda T, Mochizuki A (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879–884
Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11:153–163
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–284
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099
Castellani R, Smith MA, Richey PL, Kalaria R, Gambetti P, Perry G (1995) Evidence for oxidative stress in Pick disease and corticobasal degeneration. Brain Res 696:268–271
Castellani R, Smith M, Richey P, Perry G (1996) Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res 737:195–200
Clayton DF, George JM (1999) Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58:120–129
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated c-MYC expression in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Neuropathol Appl Neurobiol 27:343–351
Forman MS, Zhukareva V, Bergeron C, Chin SS, Grossman M, Clark C, Lee VM, Trojanowski JQ (2002) Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol 160:2045–2053
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037
Gearing M, Olson DA, Watts RL, Mirra SS (1994) Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology 44:1015–1024
Gerst JL, Siedlak SL, Nunomura A, Castellani R, Perry G, Smith MA (1999) Role of oxidative stress in frontotemporal dementia. Dement Geriatr Cogn Disord 10:85–87
Ghetti B, Murrell JR, Zolo P, Spillantini MG, Goedert M (2000) Progress in hereditary tauopathies: a mutation in the Tau gene (G389R) causes a Pick disease-like syndrome. Ann N Y Acad Sci 920:52–62
Hardy J (2003) The relationship between amyloid and tau. J Mol Neurosci 20:203–206
Hashimoto M, Masliah E (2003) Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem Res 28:1743–1756
Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C (1990) Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119:182–186
Hodges JR (2001) Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology 56: S6–10
Hogg M, Grujic ZM, Baker M, Demirci S, Guillozet AL, Sweet AP, Herzog LL, Weintraub S, Mesulam MM, LaPointe NE, Gamblin TC, Berry RW, Binder LI, Silva R de, Lees A, Espinoza M, Davies P, Grover A, Sahara N, Ishizawa T, Dickson D, Yen SH, Hutton M, Bigio EH (2003) The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol 106:323–336
Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19: S85–91
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 62:389–397
Jellinger KA, Stadelmann C (2000) Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl 59:95–114
Klucken J, McLean PJ, Gomez-Tortosa E, Ingelsson M, Hyman BT (2003) Neuritic alterations and neural system dysfunction in Alzheimer’s disease and dementia with Lewy bodies. Neurochem Res 28:1683–1691
Krishnamurthy PK, Johnson GV (2004) Mutant (R406W) human tau is hyperphosphorylated and does not efficiently bind microtubules in a neuronal cortical cell model. J Biol Chem 279:7893–7900
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Lipton AM, White CL 3rd, Bigio EH (2004) Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 108:379–385
McKeith IG, Galaski D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe DM, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, Vos RAI de, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clincial and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 47:1113–1124
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: Report of the work group on frontotemporal dementia and Pick’s disease. Arch Neurol 58:1803–1809
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L (1991) The consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A (1988) Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull 24:641–652
Neary D, Snowden JS, Mann DM (2000) Classification and description of frontotemporal dementias. Ann N Y Acad Sci 920:46–51
Neumann M, Schulz-Schaeffer W, Crowther RA, Smith MJ, Spillantini MG, Goedert M, Kretzschmar HA (2001) Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol 50:503–513
Pickering-Brown S, Baker M, Yen SH, Liu WK, Hasegawa M, Cairns N, Lantos PL, Rossor M, Iwatsubo T, Davies Y, Allsop D, Furlong R, Owen F, Hardy J, Mann D, Hutton M (2000) Pick’s disease is associated with mutations in the tau gene. Ann Neurol 48:859–867
Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C (2002) The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci 959:93–107
Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG (1996) Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol 92:588–596
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130
Rizzini C, Goedert M, Hodges JR, Smith MJ, Jakes R, Hills R, Xuereb JH, Crowther RA, Spillantini MG (2000) Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J Neuropathol Exp Neurol 59:990–1001
Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, Cribbs DH (2001) Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol 158:189–198
Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E (2002) Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis 11:341–354
Rohn TT, Rissman RA, Head E, Cotman CW (2002) Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect 15:549–557
Rosso SM, Herpen E van, Deelen W, Kamphorst W, Severijnen LA, Willemsen R, Ravid R, Niermeijer MF, Dooijes D, Smith MJ, Goedert M, Heutink P, Swieten JC van (2002) A novel tau mutation, S320F, causes a tauopathy with inclusions similar to those in Pick’s disease. Ann Neurol 51:373–376
Roth KA (2001) Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 60:829–838
Selznick LA, Holtzman DM, Han BH, Gokden M, Srinivasan AN, Johnson EM Jr, Roth KA (1999) In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J Neuropathol Exp Neurol 58:1020–1026
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Spillantini MG, Bird TD, Ghetti B (1998) Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 8:387–402
Stanford PM, Shepherd CE, Halliday GM, Brooks WS, Schofield PW, Brodaty H, Martins RN, Kwok JB, Schofield PR (2003) Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain 126:814–826
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Tolnay M, Probst A (1999) Review: tau protein pathology in Alzheimer’s disease and related disorders. Neuropathol Appl Neurobiol 25:171–187
Uchihara T, Mitani K, Mori H, Kondo H, Yamada M, Ikeda K (1994) Abnormal cytoskeletal pathology peculiar to corticobasal degeneration is different from that of Alzheimer’s disease or progressive supranuclear palsy. Acta Neuropathol 88:379–383
Wang B, Larson L (1985) Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Histochemistry 83:47–56
Zarkovic K (2003) 4-Hydroxynonenal and neurodegenerative diseases. Mol Aspects Med 24:293–303
Zhao Z, Ho L, Suh J, Qin W, Pyo H, Pompl P, Ksiezak-Reding H, Pasinetti GM (2003) A role of P301L tau mutant in anti-apoptotic gene expression, cell cycle and apoptosis. Mol Cell Neurosci 24:367–379
Acknowledgements
This work was supported by NIH/NIA grants R15 AG19642, P20 RR16454 from the BRIN/INBRE Program of the National Center for Research Resources, (T.T.R.), P50 AG16573 (C.W.C.), F32 AG023433 (R.A.R.), and P30 AG10161 (D.A.B.). We also thank Julie Schneider, M.D. (Rush Alzheimer’s Disease Center, Chicago, IL) for neuropathology data and MaryAnn Hill, Ph.D. for statistical consulting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newman, J., Rissman, R.A., Sarsoza, F. et al. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and α-synuclein pathology. Acta Neuropathol 110, 135–144 (2005). https://doi.org/10.1007/s00401-005-1027-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-1027-3