Abstract
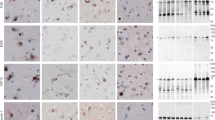
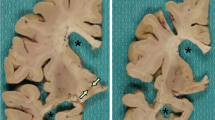
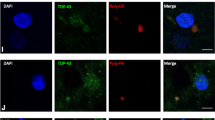
Neuronal intermediate filament inclusion disease (NIFID) is an uncommon neurodegenerative condition that typically presents as early-onset, sporadic frontotemporal dementia (FTD), associated with a pyramidal and/or extrapyramidal movement disorder. The neuropathology is characterized by frontotemporal lobar degeneration with neuronal inclusions that are immunoreactive for all class IV intermediate filaments (IF), light, medium and heavy neurofilament subunits and α-internexin. However, not all the inclusions in NIFID are IF-positive and the primary molecular defect remains uncertain. Mutations in the gene encoding the fused in sarcoma (FUS) protein have recently been identified as a cause of familial amyotrophic lateral sclerosis (ALS). Because of the recognized clinical, genetic and pathological overlap between FTD and ALS, we investigated the possible role of FUS in NIFID. We found abnormal intracellular accumulation of FUS to be a consistent feature of our NIFID cases (n = 5). More neuronal inclusions were labeled using FUS immunohistochemistry than for IF. Several types of inclusions were consistently FUS-positive but IF-negative, including neuronal intranuclear inclusions and glial cytoplasmic inclusions. Double-label immunofluorescence confirmed that many cells had only FUS-positive inclusions and that all cells with IF-positive inclusions also contained pathological FUS. No mutation in the FUS gene was identified in a single case with DNA available. These findings suggest that FUS may play an important role in the pathogenesis of NIFID.




Similar content being viewed by others
References
Al Chalabi A, Miller CC (2003) Neurofilaments and neurological disease. Bioessays 25:346–355
Aman P, Panagopoulos I, Lassen C et al (1996) Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS. Genomics 37:1–8
Andersson MK, Stahlberg A, Arvidsson Y et al (2008) The multifunctional FUS, EWS, and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol 9:37
Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT (1999) Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLD/FUS and is able to promote D-loop formation. J Biol Chem 274:34337–34342
Bertrand P, Akhmedov AT, Delacote F, Durrbach A, Lopez BS (1999) Human POMp75 is identified as the pro-oncoprotein TLS/FUS: both POMp75 and POMp100 DNA homologous pairing activities are associated with cell proliferation. Oncogene 18:4515–4521
Bigio EH, Lipton AM, White CL, Dickson DW, Hirano A (2003) Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol 29:239–253
Cairns NJ, Bigio EH, Mackenzie IRA et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Cairns NJ, Grossman M, Arnold SE et al (2004) Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63:1376–1384
Cairns NJ, Perry RH, Jaros E et al (2003) Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci Lett 341:177–180
Cairns NJ, Uryu K, Bigio EH et al (2004) α-Internexin aggregates are abundant in neuronal intermediate filament inclusion disease (NIFID) but rare in other neurodegenerative diseases. Acta Neuropathol (Berl) 108:213–223
Cairns NJ, Zhukareva V, Uryu K et al (2004) α-Internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol 164:2153–2161
Crozat A, Aman P, Mandahl N, Ron D (1993) Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 363:640–644
Doi H, Okamura K, Bauer PO et al (2008) RNA-binding protein TLS is a major nuclear aggregate-interacting protein in Huntingtin exon 1 with expanded polyglutamine-expressing cells. J Biol Chem 283:6489–6500
Duyckaerts C, Mokhtari K, Fontaine B et al (2003) Maladie de Pick généralisée: une démence mal nommée caractérisée par des inclusions neurofilamentaires. Rev Neurol 159:219
Fujii R, Okabe S, Urushido T et al (2005) The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol 15:587–593
Fujii R, Takumi T (2005) TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci 118:5755–5765
Gearing M, Castellano AA, Hunter SB et al (2003) Unusual neuropathological findings in a case of primary lateral sclerosis. J Neuropathol Exp Neurol 62:555
Josephs KA, Holton JL, Rossor MN et al (2003) Neurofilament inclusion body disease: a new proteinopathy? Brain 126:2291–2303
Josephs KA, Uchikado H, McComb RD et al (2005) Extending the clinicopathological spectrum of neurofilament inclusion disease. Acta Neuropathol 109:427–432
Kwiatkowski TJ, Bosco DA, LeClerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208
Lagier-Tourenne C, Cleveland DW (2009) Rethinking ALS: the FUS about TDP-43. Cell 136:1001–1004
Law WJ, Cann KL, Hicks GG (2006) TLS, EWS, and TAF15: a model for transcriptional integration of gene expression. Brief Funct Genomic Proteomic 5:8–14
Liu Q, Xie F, Siedlak SL et al (2004) Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci 61:3057–3075
Mackenzie IR, Feldman H (2004) Neurofilament inclusion body disease with early onset frontotemporal dementia and primary lateral sclerosis. Clin Neuropathol 23:183–193
Mackenzie IRA, Foti D, Woulfe J, Hurwitz TA (2008) Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131:1282–1293
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Molina-Porcel L, Llado A, Rey MJ et al (2008) Clinical and pathological heterogeneity of neuronal intermediate filament inclusion disease. Arch Neurol 65:272–275
Momeni P, Cairns NJ, Perry RH et al (2006) Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID). Neurobiol Aging 27:778.e1–778.e6
Mosaheb S, Thorpe JR, Hashemzadeh-Bonehi L, Bigio EH, Gearing M, Cairns NJ (2005) Neuronal intranuclear inclusions are ultrastructurally and immunologically distinct from cytoplasmic inclusions of neuronal intermediate filament inclusion disease. Acta Neuropathol 110:360–368
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA (2009) Frontotemporal lobar degeneration with FUS pathology. Brain. doi:10.1093/brain/awp214
Perrotti D, Bonatti S, Trotta R et al (1998) TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J 17:4442–4455
Roeber S, Bazner H, Hennerici M, Porstmann R, Kretzschmar HA (2006) Neurodegeneration with features of NIFID and ALS–extended clinical and neuropathological spectrum. Brain Pathol 16:228–234
Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M (2008) TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol 116:147–157
Trojanowski JQ, Dickson D (2001) Update on the neuropathological diagnosis of frontotemporal dementia. J Neuropathol Exp Neurol 60:1123–1126
Uchikado H, Li A, Lin WL, Dickson DW (2006) Heterogeneous inclusions in neurofilament inclusion disease. Neuropathol 26:417–421
Uchikado H, Shaw G, Wang DS, Dickson DW (2005) Screening for neurofilament inclusion disease using alpha-internexin immunohistochemistry. Neurology 64:1658–1659
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Yang L, Embree LJ, Tsai S, Hickstein DD (1998) Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing. J Biol Chem 273:27761–27764
Yokoo H, Oyama T, Hirato J, Sasaki A, Nakazato Y (1994) A case of Pick’s disease with unusual neuronal inclusions. Acta Neuropathol 88:267–272
Yokota O, Tsuchiya K, Terada S et al (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Zinszner H, Sok J, Immanuel D, Ron D (1997) TLD (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci 110:1741–1750
Acknowledgments
We thank Margaret Luk, Mareike Schroff and Mirjam Lutz for their excellent technical assistance. This work was supported by grants from Canadian Institutes of Health Research (grant number 74580, IM); the Pacific Alzheimer Research Foundation (IM); the Deutsche Forschungsgemeinschaft (SFB 596, MN); the Stavros-Niarchos Foundation (MN); the Synapsis Foundation (MN); the German Brain Bank “BrainNet” (HK) and the National Institute of Health (grant number P50 AG16574, RR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neumann, M., Roeber, S., Kretzschmar, H.A. et al. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 118, 605–616 (2009). https://doi.org/10.1007/s00401-009-0581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0581-5