Abstract
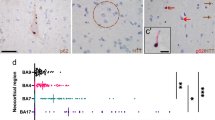
We report a retrospective case series of four patients with genetically confirmed Huntington’s disease (HD) and sporadic amyotrophic lateral sclerosis (ALS), examining the brain and spinal cord in two cases. Neuropathological assessment included a polyglutamine recruitment method to detect sites of active polyglutamine aggregation, and biochemical and immunohistochemical assessment of TDP-43 pathology. The clinical sequence of HD and ALS varied, with the onset of ALS occurring after the mid-50’s in all cases. Neuropathologic features of HD and ALS coexisted in both cases examined pathologically: neuronal loss and gliosis in the neostriatum and upper and lower motor neurons, with Bunina bodies and ubiquitin-immunoreactive skein-like inclusions in remaining lower motor neurons. One case showed relatively early HD pathology while the other was advanced. Expanded polyglutamine-immunoreactive inclusions and TDP-43-immunoreactive inclusions were widespread in many regions of the CNS, including the motor cortex and spinal anterior horn. Although these two different proteinaceous inclusions coexisted in a small number of neurons, the two proteins did not co-localize within inclusions. The regional distribution of TDP-43-immunoreactive inclusions in the cerebral cortex partly overlapped with that of expanded polyglutamine-immunoreactive inclusions. In the one case examined by TDP-43 immunoblotting, similar TDP-43 isoforms were observed as in ALS. Our findings suggest the possibility that a rare subset of older HD patients is prone to develop features of ALS with an atypical TDP-43 distribution that resembles that of aggregated mutant huntingtin. Age-dependent neuronal dysfunction induced by mutant polyglutamine protein expression may contribute to later-life development of TDP-43 associated motor neuron disease in a small subset of patients with HD.




Similar content being viewed by others
References
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Da Cruz S, Cleveland DW (2011) Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol 21:904–919
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Elden AC, Kim HJ, Hart MP et al (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466:1069–1075
Furtado S, Payami H, Lockhart PJ et al (2004) Profile of families with parkinsonism-predominant spinocerebellar ataxia type 2 (SCA2). Mov Disord 19:622–629
Gatchel JR, Zoghbi HY (2005) Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet 6:743–755
Geser F, Martinez-Lage M, Robinson J et al (2009) Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 66:180–189
Hasegawa M, Arai T, Nonaka T et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Hedreen JC, Roos RAC (2003) Huntington’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 229–241
Heng MY, Duong DK, Albin RL et al (2010) Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 19:3702–3720
Herndon ES, Hladik CL, Shang P, Burns DK, Raisanen J, White CL 3rd (2009) Neuroanatomic profile of polyglutamine immunoreactivity in Huntington disease brains. J Neuropathol Exp Neurol 68:250–261
Infante J, Berciano J, Volpini V et al (2004) Spinocerebellar ataxia type 2 with Levodopa-responsive Parkinsonism culminating in motor neuron disease. Mov Disord 19:848–852
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574
Kanai K, Kuwabara S, Sawai S et al (2008) Genetically confirmed Huntington’s disease masquerading as motor neuron disease. Mov Disord 23:748–751
Kato S, Shaw P, Wood-Allum C, Leigh PN (2003) Amyotrophic lateral sclerosis. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 350–368
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955
Lee T, Li YR, Chesi A et al (2011) Evaluating the prevalence of polyglutamine repeat expansions in amyotrophic lateral sclerosis. Neurology 76:2062–2065
Mackenzie IR, Baborie A, Pickering-Brown S et al (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD–TDP pathology. Acta Neuropathol 122:111–113
Nanetti L, Fancellu R, Tomasello C, Gellera C, Pareyson D, Mariotti C (2009) Rare association of motor neuron disease and spinocerebellar ataxia type 2 (SCA2): a new case and review of the literature. J Neurol 256:1926–1928
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Ohara S, Iwahashi T, Oide T et al (2002) Spinocerebellar ataxia type 6 with motor neuron loss: a follow-up autopsy report. J Neurol 249:633–635
Osmand AP, Berthelier V, Wetzel R (2006) Imaging polyglutamine deposits in brain tissue. Methods Enzymol 412:106–122
Papageorgiou SG, Antelli A, Bonakis A et al (2006) Association of genetically proven Huntington’s disease and sporadic amyotrophic lateral sclerosis in a 72-year-old woman. J Neurol 253:1649–1650
Paulson HL (2007) Dominantly inherited ataxias: lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin Neurol 27:133–142
Phukan J, Ali E, Pender NP et al (2010) Huntington’s disease presenting as amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11:405–407
Piao YS, Wakabayashi K, Kakita A et al (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Ramos EM, Keagle P, Gillis T et al (2012) Prevalence of Huntington’s disease gene CAG repeat alleles in sporadic amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler 13:265–269
Ross OA, Rutherford NJ, Baker M et al (2011) Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet 20:3207–3212
Rubio A, Steinberg K, Figlewicz DA et al (1996) Coexistence of Huntington’s disease and familial amyotrophic lateral sclerosis: case presentation. Acta Neuropathol 92:421–427
Sadeghian H, O’Suilleabhain PE, Battiste J, Elliott JL, Trivedi JR (2011) Huntington chorea presenting with motor neuron disease. Arch Neurol 68:650–652
Sampathu DM, Neumann M, Kwong LK et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL (2008) Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol 67:1159–1165
Seidel K, den Dunnen WF, Schultz C et al (2010) Axonal inclusions in spinocerebellar ataxia type 3. Acta Neuropathol 120:449–460
Sobue G, Adachi H, Katsuno M (2003) Spinal and bulbar muscular atrophy. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 275–279
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Tan CF, Yamada M, Toyoshima Y et al (2009) Selective occurrence of TDP-43-immunoreactive inclusions in the lower motor neurons in Machado-Joseph disease. Acta Neuropathol 118:553–560
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Toyoshima Y, Tanaka H, Shimohata M et al (2011) Spinocerebellar ataxia type 2 (SCA2) is associated with TDP-43 pathology. Acta Neuropathol 122:375–378
Van Damme P, Veldink JH, van Blitterswijk M et al (2011) Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology 76:2066–2072
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Yamada M, Tsuji S, Takahashi H (2000) Pathology of CAG repeat diseases. Neuropathology 20:319–325
Yokoseki A, Shiga A, Tan CF et al (2008) TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63:538–542
Acknowledgments
This work was funded by NIH RO1 NS 038712 and RO1 AG034228 (HLP). A grant was received from Hereditary Disease Foundation (APO), Research Committee for CNS Degenerative Diseases, the Ministry of Health, Labour and Welfare, Japan and a Grant-in-Aid (23240049) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (HT).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tada, M., Coon, E.A., Osmand, A.P. et al. Coexistence of Huntington’s disease and amyotrophic lateral sclerosis: a clinicopathologic study. Acta Neuropathol 124, 749–760 (2012). https://doi.org/10.1007/s00401-012-1005-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1005-5