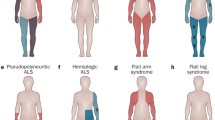
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of motor neurons leading to death from respiratory failure within about 3 years of symptom onset. A family history of ALS is obtained in about 5 % but the distinction between familial and apparently sporadic ALS is artificial and genetic factors play a role in all types. For several years, only one gene was known to have a role in ALS pathogenesis, SOD1. In the last few years there has been a rapid advance in our genetic knowledge of the causes of ALS, and the relationship of the genetic subtypes with pathological subtypes and clinical phenotype. Mutations in the gene for TDP-43 protein, TARDBP, highlight this, with pathology mimicking closely that found in other types of ALS, and a phenotypic spectrum that includes frontotemporal dementia. Mutations in the FUS gene, closely related to TDP-43, lead to a similar clinical phenotype but distinct pathology, so that the three pathological groups represented by SOD1, TARDBP, and FUS are distinct. In this review, we explore the genetic architecture of ALS, highlight some of the genes implicated in pathogenesis, and describe their phenotypic range and overlap with other diseases.




Similar content being viewed by others
References
Al-Chalabi A, Andersen PM, Nilsson P et al (1999) Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet 8:157–164. doi:10.1093/hmg/8.2.157
Al-Chalabi A, Fang F, Hanby MF et al (2010) An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry 81:1324–1326. doi:10.1136/jnnp.2010.207464
Al-Chalabi A, Lewis CM (2011) Modelling the effects of penetrance and family size on rates of sporadic and familial disease. Hum Hered 71:281–288. doi:10.1159/000330167
Al-Saif A, Al-Mohanna F, Bohlega S (2011) A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol 70:913–919. doi:10.1002/ana.22534
Al-Sarraj S, King A, Troakes C et al (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122:691–702. doi:10.1007/s00401-011-0911-2
Andersen PM, Nilsson P, Ala-Hurula V et al (1995) Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet 10:61–66. doi:10.1038/ng0595-61
Andersen PM, Al-Chalabi A (2011) Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 7:603–615. doi:10.1038/nrneurol.2011.150
Beckman JS, Carson M, Smith CD, Koppenol WH (1993) ALS, SOD and peroxynitrite. Nature 364:584
Bosco DA, Morfini G, Karabacak NM et al (2010) Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci 13:1396–1403. http://www.nature.com/neuro/journal/v13/n11/abs/nn.2660.html#supplementary-information
Boxer AL, Mackenzie IR, Boeve BF et al (2009) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp.2009.204081
Boxer AL, Mackenzie IR, Boeve BF et al (2011) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatr. doi:10.1136/jnnp.2009.204081
Brotherton T, Polak M, Kelly C et al (2011) A novel ALS SOD1 C6S mutation with implications for aggregation related toxicity and genetic counseling. Amyotroph Later Scler Off Publ World Fed Neurol Res Group Motor Neuron Dis 12:215–219. doi:10.3109/17482968.2010.531279
Byrne S, Hardiman O (2010) Familial aggregation in amyotrophic lateral sclerosis. Ann Neurol 67:554. doi:10.1002/ana.21883
Byrne S, Bede P, Elamin M et al (2011) Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Later Scler Off Publ World Fed Neurol Res Group Motor Neuron Dis 12:157–159. doi:10.3109/17482968.2010.545420
Byrne S, Walsh C, Lynch C et al (2011) Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatr 82:623–627. doi:10.1136/jnnp.2010.224501
Byrne S, Elamin M, Bede P et al (2012) Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. doi:10.1016/S1474-4422(12)70014-5
Chen Y, Bennett C, Huynh H et al (2004) DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet 74:1128–1135
Chen Y, Huang R, Yang Y et al (2011) Ataxin-2 intermediate-length polyglutamine: a possible risk factor for Chinese patients with amyotrophic lateral sclerosis. Neurobiol Aging 32(1925):e1921–e1925. doi:10.1016/j.neurobiolaging.2011.05.015
Chio A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R (2009) Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology 72:725–731. doi:10.1212/01.wnl.0000343008.26874.d1
Chow CY, Landers JE, Bergren SK et al (2009) Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet 84:85–88
Corrado L, Carlomagno Y, Falasco L et al (2011) A novel peripherin gene (PRPH) mutation identified in one sporadic amyotrophic lateral sclerosis patient. Neurobiol Aging 32:552.e551–552.e556. doi:10.1016/j.neurobiolaging.2010.02.011
Couthouis J, Hart MP, Shorter J et al (2011) A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci USA 108:20881–20890. doi:10.1073/pnas.1109434108
Couthouis J, Hart MP, Erion R et al (2012) Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum Mol Genet 21:2899–2911. doi:10.1093/hmg/dds116
Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS (1997) Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem 69:1936–1944. doi:10.1046/j.1471-4159.1997.69051936.x
Daoud H, Belzil V, Martins S et al (2011) Association of long ATXN2 CAG repeat sizes with increased risk of amyotrophic lateral sclerosis. Arch Neurol 68:739–742. doi:10.1001/archneurol.2011.111
DeJesus-Hernandez M, Desaro P, Johnston A et al (2011) Novel p.Ile151Val mutation in VCP in a patient of African American descent with sporadic ALS. Neurology 77:1102–1103. doi:10.1212/WNL.0b013e31822e563c
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. doi:10.1016/j.neuron.2011.09.011
Deng HX, Zhai H, Bigio EH et al (2010) FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol 67:739–748. doi:10.1002/ana.22051
Deng HX, Chen W, Hong ST et al (2011) Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477:211–215. doi:10.1038/nature10353
Devon RS, Helm JR, Rouleau GA et al (2003) The first nonsense mutation in alsin results in a homogeneous phenotype of infantile-onset ascending spastic paralysis with bulbar involvement in two siblings. Clin Genet 64:210–215
Diekstra FP, van Vught PW, van Rheenen W et al (2012) UNC13A is a modifier of survival in amyotrophic lateral sclerosis. Neurobiol Aging 33(630):e633–e638. doi:10.1016/j.neurobiolaging.2011.10.029
Dormann D, Rodde R, Edbauer D et al (2010) ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J 29:2841–2857. http://www.nature.com/emboj/journal/v29/n16/suppinfo/emboj2010143a_S1.html
Elden AC, Kim H-J, Hart MP et al (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466:1069–1075. http://www.nature.com/nature/journal/v466/n7310/abs/nature09320.html#supplementary-information
Eymard-Pierre E, Lesca G, Dollet S et al (2002) Infantile-onset ascending hereditary spastic paralysis is associated with mutations in the alsin gene. Am J Hum Genet 71:518–527. doi:10.1086/342359
Fecto F, Siddique T (2012) UBQLN2/P62 cellular recycling pathways in amyotrophic lateral sclerosis and frontotemporal dementia. Muscle Nerve 45:157–162. doi:10.1002/mus.23278
Figlewicz DA, Krizus A, Martinoli MG et al (1994) Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet 3:1757–1761
Garcia ML, Singleton AB, Hernandez D et al (2006) Mutations in neurofilament genes are not a significant primary cause of non-SOD1-mediated amyotrophic lateral sclerosis. Neurobiol Dis 21:102–109. doi:10.1016/j.nbd.2005.06.016
Gijselinck I, Van Langenhove T, van der Zee J et al (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11:54–65. doi:10.1016/S1474-4422(11)70261-7
Gispert S, Kurz A, Waibel S et al (2012) The modulation of amyotrophic lateral sclerosis risk by ataxin-2 intermediate polyglutamine expansions is a specific effect. Neurobiol Dis 45:356–361. doi:10.1016/j.nbd.2011.08.021
Gitcho MA, Baloh RH, Chakraverty S et al (2008) TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol 63:535–538. doi:10.1002/ana.21344
Goodall EF, Greenway MJ, van Marion I, Carroll CB, Hardiman O, Morrison KE (2005) Association of the H63D polymorphism in the hemochromatosis gene with sporadic ALS. Neurology 65:934–937. doi:10.1212/01.wnl.0000176032.94434.d4
Greenway MJ, Andersen PM, Russ C et al (2006) ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet 38:411–413. doi:10.1038/ng1742
Gros-Louis F, Larivière R, Gowing G et al (2004) A frameshift deletion in peripherin gene associated with amyotrophic lateral sclerosis. J Biol Chem 279:45951–45956. doi:10.1074/jbc.M408139200
Gunnarsson LG, Dahlbom K, Strandman E (1991) Motor neuron disease and dementia reported among 13 members of a single family. Acta Neurol Scand 84:429–433
Gurney M, Pu H, Chiu A et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775. doi:10.1126/science.8209258
Hadano S, Hand C, Osuga H et al (2001) A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet 29:166–173
Hanby MF, Scott KM, Scotton W et al (2011) The risk to relatives of patients with sporadic amyotrophic lateral sclerosis. Brain J Neurol 134:3454–3457. doi:10.1093/brain/awr248
Hayward C, Colville S, Swingler RJ, Brock DJ (1999) Molecular genetic analysis of the APEX nuclease gene in amyotrophic lateral sclerosis. Neurology 52:1899–1901
Higgins CMJ, Jung C, Ding H, Xu Z (2002) Mutant Cu, Zn Superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci 22:RC215
Hortobagyi T, Troakes C, Nishimura AL et al (2011) Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD-TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol 121:519–527. doi:10.1007/s00401-011-0813-3
Huisman MH, de Jong SW, Verwijs MC et al (2011) Family history of neurodegenerative and vascular diseases in ALS: a population-based study. Neurology 77:1363–1369. doi:10.1212/WNL.0b013e318231530b
Johnson JO, Mandrioli J, Benatar M et al (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864
Johnston CA, Stanton BR, Turner MR et al (2006) Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol 253:1642–1643. doi:10.1007/s00415-006-0195-y
Julien JP, Côté F, Collard JF (1995) Mice overexpressing the human neurofilament heavy gene as a model of ALS. Neurobiol Aging 16:487–490. doi:10.1016/0197-4580(94)00169-2
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574. doi:http://www.nature.com/ng/journal/v40/n5/suppinfo/ng.132_S1.html
King A, Maekawa S, Bodi I, Troakes C, Al-Sarraj S (2011) Ubiquitinated, p62 immunopositive cerebellar cortical neuronal inclusions are evident across the spectrum of TDP-43 proteinopathies but are only rarely additionally immunopositive for phosphorylation-dependent TDP-43. Neuropathol Off J Jpn Soc Neuropathol 31:239–249. doi:10.1111/j.1440-1789.2010.01171.x
Koppers M, van Blitterswijk MM, Vlam L et al (2011) VCP mutations in familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.10.006
Kwiatkowski TJ Jr, Bosco DA, LeClerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208. doi:10.1126/science.1166066
Laaksovirta H, Peuralinna T, Schymick JC et al (2010) Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol 9:978–985. doi:10.1016/S1474-4422(10)70184-8
Lagier-Tourenne C, Cleveland DW (2009) Rethinking ALS: the FUS about TDP-43. Cell 136:1001–1004. doi:10.1016/j.cell.2009.03.006
Lambrechts D, Storkebaum E, Morimoto M et al (2003) VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet 34:383–394. doi:10.1038/ng1211ng1211
Landers JE, Leclerc AL, Shi L et al (2008) New VAPB deletion variant and exclusion of VAPB mutations in familial ALS. Neurology 70:1179–1185. doi:10.1212/01.wnl.0000289760.85237.4e
Landers JE, Melki J, Meininger V et al (2009) Reduced expression of the kinesin-associated protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 106:9004–9009. doi:10.1073/pnas.0812937106
Lansbury PT, Lashuel HA (2006) A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443:774–779
Leigh P, Whitwell H, Garofalo O et al (1991) Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain J Neurol 114:775–788
Leigh PN, Dodson A, Swash M, Brion JP, Anderton BH (1989) Cytoskeletal abnormalities in motor neuron disease. An immunocytochemical study. Brain J Neurol 112(Pt 2):521–535
Lesca G, Eymard-Pierre E, Santorelli FM et al (2003) Infantile ascending hereditary spastic paralysis (IAHSP): clinical features in 11 families. Neurology 60:674–682
Leung CL, He CZ, Kaufmann P et al (2004) A pathogenic peripherin gene mutation in a patient with amyotrophic lateral sclerosis. Brain Pathol 14:290–296. doi:10.1111/j.1750-3639.2004.tb00066.x
Ligon LA, LaMonte BH, Wallace KE, Weber N, Kalb RG, Holzbaur ELFCA (2005) Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. NeuroReport 16:533–536
Lill CM, Abel O, Bertram L, Al-Chalabi A (2011) Keeping up with genetic discoveries in amyotrophic lateral sclerosis: the ALSoD and ALSGene databases. Amyotroph Later Scler Off Publ World Feder Neurol Res Group Motor Neuron Dis 12:238–249. doi:10.3109/17482968.2011.584629
Mackenzie I, Bigio E, Ince P et al (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Mackenzie IR, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9:995–1007. doi:10.1016/S1474-4422(10)70195-2
Martinaud O, Laquerrière A, Guyant-Maréchal L et al (2005) Frontotemporal dementia, motor neuron disease and tauopathy: clinical and neuropathological study in a family. Acta Neuropathol 110:84–92. doi:10.1007/s00401-005-1028-2
Maruyama H, Morino H, Ito H et al (2010) Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465:223–226. http://www.nature.com/nature/journal/v465/n7295/suppinfo/nature08971_S1.html
Mitchell J, Paul P, Chen H-J et al (2010) Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Nat Acad Sci USA 107:7556–7561. doi:10.1073/pnas.0914128107
Morita M, Al-Chalabi A, Andersen PM et al (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844. doi:10.1212/01.wnl.0000200048.53766.b4
Murray ME, DeJesus-Hernandez M, Rutherford NJ et al (2011) Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122:673–690. doi:10.1007/s00401-011-0907-y
Nagai M, Re DB, Nagata T et al (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10:615–622. http://www.nature.com/neuro/journal/v10/n5/suppinfo/nn1876_S1.html
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA (2009) A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain J Neurol 132:2922–2931. doi:10.1093/brain/awp214
Neumann M, Bentmann E, Dormann D et al (2011) FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain J Neurol 134:2595–2609. doi:10.1093/brain/awr201
Nishimura AL, Mitne-Neto M, Silva HC et al (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75:822–831. doi:10.1086/425287S0002-9297(07)63787-2
Nishimura AL, Al-Chalabi A, Zatz M (2005) A common founder for amyotrophic lateral sclerosis type 8 (ALS8) in the Brazilian population. Hum Genet 118:499–500. doi:10.1007/s00439-005-0031-y
Niwa J-I, Yamada S-I, Ishigaki S et al (2007) Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem 282:28087–28095. doi:10.1074/jbc.M704465200
Orlacchio A, Babalini C, Borreca A et al (2010) SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain J Neurol 133:591–598. doi:10.1093/brain/awp325
Orsetti V, Pegoraro E, Cima V et al (2011) Genetic variation in KIFAP3 is associated with an upper motor neuron-predominant phenotype in amyotrophic lateral sclerosis. Neurodegener Dis 8:491–495. doi:10.1159/000327755
Osawa T, Mizuno Y, Fujita Y, Takatama M, Nakazato Y, Okamoto K (2011) Optineurin in neurodegenerative diseases. Neuropathol Off J Jpn Soc Neuropathol 31:569–574. doi:10.1111/j.1440-1789.2011.01199.x
Page T, Gitcho M, Mosaheb S et al (2011) FUS immunogold labeling TEM analysis of the neuronal cytoplasmic inclusions of neuronal intermediate filament inclusion disease: a frontotemporal lobar degeneration with FUS proteinopathy. J Mol Neurosci 45:409–421. doi:10.1007/s12031-011-9549-8
Parkinson N, Ince PG, Smith MO et al (2006) ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67:1074–1077. doi:10.1212/01.wnl.0000231510.89311.8b
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7:710–723. http://www.nature.com/nrn/journal/v7/n9/suppinfo/nrn1971_S1.html
Pearson JP, Williams NM, Majounie E et al (2011) Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol 258:647–655. doi:10.1007/s00415-010-5815-x
Praline J, Blasco H, Vourc’h P et al (2012) Study of the HFE gene common polymorphisms in French patients with sporadic amyotrophic lateral sclerosis. J Neurol Sci 317:58–61. doi:10.1016/j.jns.2012.02.029
Puls I, Jonnakuty C, LaMonte B et al (2003) Mutant dynactin in motor neuron disease. Nat Genet 33:455–456
Renton AE, Majounie E, Waite A et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. doi:10.1016/j.neuron.2011.09.010
Rooke K, Figlewicz DA, Han FY, Rouleau GA (1996) Analysis of the KSP repeat of the neurofilament heavy subunit in familiar amyotrophic lateral sclerosis. Neurology 46:789–790
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. doi:10.1038/362059a0
Ross OA, Rutherford NJ, Baker M et al (2011) Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet 20:3207–3212. doi:10.1093/hmg/ddr227
Rouleau GA, Clark AW, Rooke K et al (1996) SOD1 mutation is assosiated with accumulation of neurofilaments in amyotrophic lateral scelaries. Ann Neurol 39:128–131. doi:10.1002/ana.410390119
Shatunov A, Mok K, Newhouse S et al (2010) Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol 9:986–994. doi:10.1016/S1474-4422(10)70197-6
Shibata N, Hirano A, Kobayashi M et al (1996) Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol 55:481–490
Simpson CL, Lemmens R, Miskiewicz K et al (2009) Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum Mol Genet 18:472–481. doi:10.1093/hmg/ddn375
Spreux-Varoquaux O, Gilbert B, Lucette L et al (2002) Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci 193:73–78
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672. doi:10.1126/science.1154584
Strong MJ, Yang W, Strong WL, Leystra-Lantz C, Jaffe H, Pant HC (2006) Tau protein hyperphosphorylation in sporadic ALS with cognitive impairment. Neurology 66:1770–1771. doi:10.1212/01.wnl.0000218161.15834.db
Sutedja NA, Sinke RJ, Van Vught PW et al (2007) The association between H63D mutations in HFE and amyotrophic lateral sclerosis in a Dutch population. Arch Neurol 64:63–67. doi:10.1001/archneur.64.1.63
Tan CF, Eguchi H, Tagawa A et al (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542. doi:10.1007/s00401-007-0206-9
Ticozzi N, Vance C, LeClerc AL et al (2011) Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am J Med Genet Part B Neuropsychiat Genet 156:285–290. doi:10.1002/ajmg.b.31158
Tiloca C, Ratti A, Pensato V et al (2012) Mutational analysis of VCP gene in familial amyotrophic lateral sclerosis. Neurobiol Aging 33(630):e631–e632. doi:10.1016/j.neurobiolaging.2011.10.025
Tomkins J, Usher P, Slade JY et al (1998) Novel insertion in the KSP region of the neurofilament heavy gene in amyotrophic lateral sclerosis (ALS). NeuroReport 9:3967–3970
Traynor BJ, Nalls M, Lai SL et al (2010) Kinesin-associated protein 3 (KIFAP3) has no effect on survival in a population-based cohort of ALS patients. Proc Natl Acad Sci USA 107:12335–12338. doi:10.1073/pnas.0914079107
Troakes C, Maekawa S, Wijesekera L et al (2011) An MND/ALS phenotype associated with C9orf72 repeat expansion: Abundant p62-positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathol Offl J Jpn Soc Neuropathol. doi:10.1111/j.1440-1789.2011.01286.x
Valdmanis PNB, Rouleau GAMDP (2008) Genetics of familial amyotrophic lateral sclerosis. Neurology 70:144–152
Van Damme P, Veldink JH, van Blitterswijk M et al (2011) Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology 76:2066–2072. doi:10.1212/WNL.0b013e31821f445b
Van Deerlin VM, Leverenz JB, Bekris LM et al (2008) TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol 7:409–416. doi:10.1016/s1474-4422(08)70071-1
Van Deerlin VM, Sleiman PM, Martinez-Lage M et al (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239. doi:10.1038/ng.536
van Es MA, Diekstra FP, Veldink JH et al (2009) A case of ALS-FTD in a large FALS pedigree with a K17I ANG mutation. Neurology 72:287–288. doi:10.1212/01.wnl.0000339487.84908.00
van Es MA, Veldink JH, Saris CG et al (2009) Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet 41:1083–1087. doi:10.1038/ng.442
van Es MA, Schelhaas HJ, van Vught PW et al (2011) Angiogenin variants in Parkinson disease and amyotrophic lateral sclerosis. Ann Neurol 70:964–973. doi:10.1002/ana.22611
Van Langenhove T, van der Zee J, Engelborghs S et al (2011) Ataxin-2 polyQ expansions in FTLD-ALS spectrum disorders in Flanders-Belgian cohorts. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.09.025
Van Swieten J, Spillantini MG (2007) Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol 17:63–73. doi:10.1111/j.1750-3639.2007.00052.x
Vance C, Al-Chalabi A, Ruddy D et al (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain J Neurol 129:868–876. doi:10.1093/brain/awl030
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211. doi:10.1126/science.1165942
Wang XS, Lee S, Simmons Z et al (2004) Increased incidence of the Hfe mutation in amyotrophic lateral sclerosis and related cellular consequences. J Neurol Sci 227:27–33. doi:10.1016/j.jns.2004.08.003
Wicks P, Abrahams S, Papps B et al (2009) SOD1 and cognitive dysfunction in familial amyotrophic lateral sclerosis. J Neurol 256:234–241. doi:10.1007/s00415-009-0078-0
Wiedau-Pazos M, Goto JJ, Rabizadeh S et al (1996) Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science 271:515–518
Williams KL, Solski JA, Nicholson GA, Blair IP (2011) Mutation analysis of VCP in familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.11.022
Williams KL, Warraich ST, Yang S et al (2012) UBQLN2/ubiquilin 2 mutation and pathology in familial amyotrophic lateral sclerosis. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2012.05.008
Yang W, Ang LC, Strong MJ (2005) Tau protein aggregation in the frontal and entorhinal cortices as a function of aging. Dev Brain Res 156:127–138. doi:10.1016/j.devbrainres.2005.02.004
Yang Y, Hentati A, Deng HX et al (2001) The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet 29:160–165. doi:10.1038/ng1001-160
Yen AA, Simpson EP, Henkel JS, Beers DR, Appel SH (2004) HFE mutations are not strongly associated with sporadic ALS. Neurology 62:1611–1612
Yokoseki A, Shiga A, Tan CF et al (2008) TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63:538–542. doi:10.1002/ana.21392
Yu Z, Zhu Y, Chen-Plotkin AS et al (2011) PolyQ repeat expansions in ATXN2 associated with ALS are CAA interrupted repeats. PLoS One 6:e17951. doi:10.1371/journal.pone.0017951
Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM (2008) Association of APOE with age at onset of sporadic amyotrophic lateral sclerosis. J Neurol Sci 273:67–69. doi:10.1016/j.jns.2008.06.025
Acknowledgments
We thank the Motor Neurone Disease Association of Great Britain and Northern Ireland, the ALS Association, the Angel Fund and the ALS Therapy Alliance for support. We thank the European Community’s Health Seventh Framework Programme (FP7/2007–2013) [259867 (to A.A.C. and L.vD.B.)]. A.A.C. receives salary support from the National Institute for Health Research (NIHR) Dementia Biomedical Research Unit at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Chalabi, A., Jones, A., Troakes, C. et al. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol 124, 339–352 (2012). https://doi.org/10.1007/s00401-012-1022-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1022-4