Abstract
Recurrent respiratory papillomatosis (RRP) is a chronic and difficult to treat disease of the larynx. In 1998, the first article was published that described the use of the antiviral substance cidofovir to treat this disease. Although the results are promising, there remains some concern about the potential carcinogenicity of cidofovir. There is a demand for a qualitative review of the side-effects of this medicine. In this review, the side-effects of cidofovir are investigated. Special attention was given to the potential carcinogenicity of cidofovir. For this review a search is performed in PubMed and EMBASE for relevant articles in which the use of intralesional cidofovir for patients with RRP is described. Eventually, 31 articles could be included for this review. In these articles a total of 188 patients with RRP were described who underwent therapy with intralesional cidofovir. Five of these patients have developed dysplasia of the larynx during the treatment with cidofovir. This is a percentage of 2.7. This percentage is concurrent with the incidence of spontaneous malignant degeneration of RRP (2–3%). Based on this review, it can be concluded that the use of intralesional cidofovir does not increase the risk of laryngeal dysplasia. Apart from the articles that describe the intralesional administration of cidofovir, some articles have been published in which the use of intravenous cidofovir is described as a therapy for RRP. Therefore, a summary is given on the side-effects of intralesional cidofovir as well as a summary on the reported side-effects of the intravenous administration of cidofovir. Based on the outcomes of this review, recommendations are given for a safe use of cidofovir for treatment of recurrent respiratory papillomatosis in the future.
Similar content being viewed by others
Introduction
Recurrent respiratory papillomatosis (RRP) is a chronic disease of the larynx which is difficult to treat. The disease is caused by an infection with human papilloma viruses (HPV). In most cases, the HPV-subtypes 6 and 11 are found. Rarely the subtypes 16 and 18 are identified [48]. The symptoms of this uncommon disease may vary from hoarseness to severe obstruction of the airway. In 1998, the first article was published that described the off-label use of cidofovir to treat RRP [51].
Cidofovir is a cytosine nucleotide analogue with antiviral activity that is approved by the US Food and Drug Administration (FDA) for the treatment of cytomegalovirus (CMV)-retinitis in persons with acquired immunodeficiency syndrome. For the treatment of CMV-retinitis the cidofovir is administered intravenously in a dose of 5 mg/kg. Since 1998 several studies have been published in which the results of cidofovir in the treatment of RRP were analysed [6, 12, 20, 30, 36, 42, 43, 45, 48]. In most of these studies the therapy with intralesional cidofovir proved to be effective.
Although few side-effects of cidofovir have been reported there is some concern about the potential carcinogenicity of cidofovir. A study in rats has shown a significant increase in mammary adenocarcinomas [24]. There have also been some reports of dysplasia in humans after the use of intralesional cidofovir [20, 48, 51, 55]. However, spontaneous malignant degeneration of RRP is possible. The reported incidence of this malignant degeneration is 2–5% [29, 51]. For these percentages no distinction was made between different HPV-subtypes. Spontaneous malignant degeneration occurs in both juvenile-onset RRP and adult-onset RRP and carries a dismal prognosis [29].
Malignant transformation happens more often in patients who have a history of smoking or radiation [5]. HPV-types 16 and 18 are considered high-risk types because they are frequently associated with severe atypia and invasive carcinoma in uterine cervical epithelium [34]. Nevertheless, several articles have been published in which only the HPV-subtypes 6 and 11 were found in patients with RRP that had undergone malignant degeneration [8, 16, 27, 31, 34, 41, 44, 49]. The role of cidofovir in malignant degeneration of RRP remains unclear. Because of the increasing use of cidofovir for RRP, there is a demand for a qualitative review of the side-effects of this drug.
In this review, a summary is presented about the reported side-effects of cidofovir. Furthermore, the reported cases of dysplasia in humans after the use of intralesional cidofovir are compared to the total number of patients treated with cidofovir that have been reported in the literature.
Materials and methods
A search was performed for articles that describe the use of intralesional cidofovir in patients with RRP. The first search was performed in PubMed (1966 to August 2007):
-
#1.
Cidofovir (laryngeal OR respiratory) AND papillomatosis
-
#2.
Cidofovir [Substance Name]
-
#3.
Papilloma [Mesh]
-
#4.
(“cidofovir” [Substance Name]) AND (“Papilloma”[Mesh])
-
#5.
#1 OR #4
Sixty-three articles were found. The following inclusion criteria were applied:
-
use of intralesional cidofovir as a primary or adjuvant therapy in the larynx in human patients with RRP;
-
description of the used dosage of cidofovir;
-
description of observed side-effects;
-
study designs have to be randomized controlled trials (RCTs), comparative studies, case series or case reports.
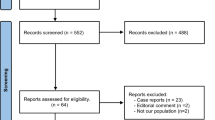
After selection with the above criteria a total of 28 articles could be included for this review.
A search in EMBASE (1966 to August 2007) was also performed:
#1. Cidofovir AND (‘laryngeal papillomatosis’ OR ‘respiratory papillomatosis’)
This way 104 articles were found from which 57 were also found in the PubMed search. The other 47 articles were screened with the above inclusion criteria. Another three articles could be included to a total of 31 articles for this review. Reference lists from relevant articles, including other reviews, were searched. No articles were excluded due to language.
The PubMed search that included 63 articles was also scanned for articles that describe other ways of application of cidofovir. Four articles were found that describe the intravenous use of cidofovir. In one article the cidofovir was nebulized.
Results
Thirty-one original articles met the original inclusion criteria. They comprised 1 case-control series, 26 case-series, 4 case-reports, and no randomized controlled trials. A summary of the articles is presented in Table 1.
Six of the 31 included articles proved to have an overlap in patient population with one of the other articles. When corrected for this overlap a total of 188 patients remained. The mean age of these patients is 26 years with a range of 1–85 years.
The patient population consisted of 98 males (52%) and 69 females (37%). In 21 patients (11%) the gender was not reported. Of this population, 104 patients (55%) were adults (above 18 years of age) and 84 patients (45%) were children (under 18 years of age). Adult-onset RRP was diagnosed in 53 patients (28%) and 89 patients (47%) were diagnosed with juvenile-onset RRP. In 46 patients (25%) it was not noted at what age the disease started.
Side-effects of cidofovir
The included articles have reported few side-effects. In the report of Snoeck et al. [51], two patients developed an immediate cutaneous rash after cidofovir injection on one occasion. It is unclear if the rash was a direct side-effect of the cidofovir. Such rashes are also known to happen to patients under total intravenous anaesthesia. Rashes were not further observed during the following sessions of treatment of these two patients.
In the same report one patient experienced a headache after each injection, which responded to symptomatic therapy. Another patient reported precordialgia on the days following each of his three injections. This patient was known for coronary insufficiency, but his electrocardiogram (ECG) did not show any changes. Inflammation, scarring or fibrosis have not been observed in any of the patients in this report.
Bielamowicz et al. [6] reported a local inflammatory response after injection with cidofovir. In this study, 14 patients received cidofovir in a concentration of 4.17–6.25 mg/ml. The inflammatory response was frequently observed during the 7th to 14th-day period after each injection. No new areas of scarring, web formation, or impaired vibration of the vocal fold mucosa were identified in this study.
In a report by Lee et al. [30] in which 13 patients were treated with cidofovir, 3 patients were found to have significant vocal fold scarring. In addition, one patient developed a supraglottic web after bilateral false vocal fold injections. The authors note that it is hard to say if the vocal fold scarring in this study should be attributed to the cidofovir or the repeated microlaryngoscopy.
One case was reported in which a compromised airway following treatment with cidofovir occurred [18]. An 8-year-old female patient developed glottis oedema after intralesional injection with cidofovir. This led to severe postoperative stenosis of the airway requiring intubation and 24 h mechanical ventilation.
One article was published in which the RRP was treated with percutaneous injection of cidofovir under local anaesthesia [9]. All patients in this study reported a mild stinging sensation as the medication was administered. There were no other complications related to the injection.
In the studies that monitored blood chemistry, no effect was noted of intralesionally injected cidofovir on haematological and chemical parameters in blood [33, 37, 38, 45, 51]. In two studies, the plasma cidofovir concentration after intralesional administration was measured [38, 51]. The used concentration of cidofovir in the first study was 2.5 mg/ml. The measured concentrations of cidofovir in plasma ranged from 0.36 to 0.6 μg/ml [51]. In the second study, a concentration of cidofovir of 7.5 mg/ml was used. The measured plasma cidofovir concentrations in this study ranged from 0.04 to 3.69 μg/ml [38]. In this last report, a linear relationship was found between plasma concentration and dose of cidofovir in children. In adults, this relationship was not found: they showed a greater diffusion than children with great individual variation. Therefore, the authors of this last report recommend that intralesional cidofovir should be used at a dose below that reported to lead to toxicity in IV use (3 mg/kg) [54].
The measured plasma cidofovir concentrations in the two above studies are remarkably lower than those achieved with systemic administration of cidofovir. In a pharmacokinetic study peak serum cidofovir concentrations of 7 and 24 μg/ml were found after cidofovir infusions of, respectively, 3 and 10 mg/kg [15].
Carcinogenicity of cidofovir
There have been some reports of dysplasia in humans after the use of intralesional cidofovir [20, 48, 51, 55]. To assess the potential carcinogenicity of cidofovir, a summary of the used dosage and concentration of cidofovir and reported dysplasia in the different articles is presented in Table 2.
A form of dysplasia has been reported in five patients. These five cases will be discussed in more detail in the section below.
In the report by Pudszuhn et al. [48], a 58-year-old female patient developed dysplasia during treatment with intralesional cidofovir. This patient had histologically confirmed adult-onset papillomatosis without known radiation or tobacco abuse. After five injections with cidofovir to a total dose of 30 mg in 4 months, a histological check-up revealed severe dysplasia of the epithelium with local transition to a carcinoma in situ. Therapy with cidofovir was discontinued. She was checked microlaryngoscopically and histologically without further carcinoma findings. In the clinical check-up 19 months after diagnosis, there was no evidence of a papilloma or malignoma. Virus typing was not performed.
In the report by Dikkers [20] a case of dysplasia was also found. From the original biopsies of this 45-year-old non-smoking male patient with adult-onset papillomatosis the HPV-subtypes 6 and 11 were identified. He received a total dose of 83.5 mg cidofovir in six injections. A biopsy 6 months post-cidofovir revealed moderate epithelial dysplasia without invasive growth. He had a pre-existent anterior glottic web, which theoretically might have hampered dilution of the injected cidofovir because of the decreased local blood flow.
Wemer et al. [55] describe the case of a 28-year-old female patient who developed dysplasia during treatment with intralesional cidofovir. Her initial pathological diagnosis was papillomatosis with mild dysplasia. She had no history of irradiation or cigarette smoking. After 17 months of treatment biopsy of a papilloma resection demonstrated moderate dysplasia. After 28 months of cidofovir-therapy biopsy demonstrated both moderate and severe dysplasia within the papillomas. Use of cidofovir was discontinued at this time. Three months after discontinuation of treatment persistence of moderate and severe dysplasia was demonstrated. All lesions submitted to biopsy were genotyped and found to be HPV-6-positive.
In the report by Snoeck et al. [51], two patients are described who showed lesions microscopically compatible with verrucous carcinoma. Both were smoking, male patients: one was 66-year-old, the other 77-year-old. They had received cidofovir for, respectively, 6.5 and 4.5 months. Additional investigation, however, has revealed that the pre-treatment biopsy specimens of these two patients showed atypical cells that may have actually been verrucous carcinoma rather than RRP [47]. Late biopsies taken from the 66-year-old patient were characterized by persistent hyperkeratosis, but in situ hybridization for HPV was negative. In the 77-year-old patient HPV typification was not performed.
In five of the 188 patients in this review, moderate to severe dysplasia has been reported during or after intralesional treatment with cidofovir. This means that in 2.7% of the patients treated with cidofovir dysplasia or malignant degeneration has occurred. This percentage is concurrent with the incidence of spontaneous malignant degeneration of RRP (2–3%). The patients in whom dysplasia has occurred consist of three adult male and two adult female patients with a mean age of 55 (28–77) years. Two patients have adult-onset RRP; in three patients was not noted when the disease started. The dysplasia was observed after an average period of 8 months of cidofovir treatment with a range of 4–17 months. In two of the five patients the cumulative dose of cidofovir is known. These two patients received amounts of 30 and 83.5 mg cidofovir. The HPV-subtype could be identified in two patients: once only subtype 6 was found and once both subtypes 6 and 11 were found.
Systemic treatment with cidofovir
Apart from the articles that describe the use of intralesional cidofovir, four articles have been published that describe the use of intravenous cidofovir as treatment for RRP [2, 17, 18, 53]. The four reported cases all had pulmonary extension of their RRP. They received the intravenous injections with cidofovir at a dose of 5 mg/kg. In all cases the intravenous injections were associated with hyperhydration and probenecid in order to diminish nephrotoxicity. From the four patients with RRP that have been treated with intravenous cidofovir one displayed side-effects. She developed leukopenia and partial alopecia on her combination therapy of cidofovir and interferon [17]. At that time she had received 34 intravenous injections with cidofovir in a period of 16 months. The therapy was well tolerated in the other three patients. They received a total of 27, 23 and 37 intravenous injections in a period of, respectively, 14, 12 and 27 months [2, 18, 53]. In the treatment of CMV-retinitis of AIDS-patients, nefrotoxicity and neutropenia have been reported as side-effects of the therapy with intravenous cidofovir [28].
Nebulized treatment with cidofovir
Apart from the four articles in which cidofovir was administered intravenously to treat the pulmonary extension of RRP, one article is published in which a case is described where the cidofovir was nebulized to reach the pulmonary lesions [26]. In this case a girl presented at 11 months of age with respiratory failure and stridor due to her RRP for which a tracheostomy was performed. To reach the lung papillomas cidofovir was nebulized through her tracheostomy tube (20 mg/ml, 4 ml, 3 times/week). Her only complication was hemoptysis that resolved with decreasing the dosage to 10 mg/ml, 4 ml, 3 times/week. Her lower airway lesions responded and diminished in size. Currently, her lower airway lesions have visually disappeared. She is maintained on cidofovir two times a week without complications.
Discussion
In the literature, three different ways to administer cidofovir as treatment for RRP have been described. In most cases, the cidofovir is administered intralesionally. Four cases have been described in which the cidofovir was administered intravenously and in one case the cidofovir was nebulized to treat RRP.
In the thirty-one articles that describe the use of intralesional cidofovir few side-effects have been reported. The only reported side-effects that have an evident causal relationship with the cidofovir injections are the local inflammatory responses in 14 adult patients described by Bielamowicz et al. [6]. The development of a supraglottic web in one patient and scarring of the vocal folds in three patients described by Lee et al. [30] could have been caused by the injections with cidofovir but could have also been caused by the repeated microlaryngoscopy. One case is described in which treatment with intralesional cidofovir in an 8-year-old girl led to glottis oedema that required intubation [18].
In two studies, the plasma cidofovir concentration after intralesional administration has been measured [38, 51]. The highest measured serum cidofovir concentration was 3.69 μg/ml. This concentration was found in a child who received a dose of 2.8 mg/kg. The concentration of 3.69 μg/ml is corresponding to a dose of 1 mg/kg in intravenous infusion [38]. As the used dose of intralesional cidofovir for the treatment of CMV-retinitis is 5 mg/kg, the serum cidofovir concentrations in these patients are remarkably higher [15]. In none of the reviewed studies a concentration higher than the recommended 3 mg/kg was administered in adults.
To assess the potential carcinogenicity of cidofovir, all the articles that describe the use of intralesional cidofovir in patients with RRP have been reviewed. This review showed that dysplasia occurred in 2.7% of all patients with RRP that have been treated with intralesional cidofovir. This percentage is concurrent with the reported incidence of spontaneous malignant degeneration of RRP (2–3%)[51]. Based on the outcomes of this review it can be concluded that the use of intralesional cidofovir does not increase the risk of laryngeal dysplasia. The two patients with verrucous carcinoma that have been described by Snoeck et al. [51] have been included in this review. However, it remains uncertain if the pre-treatment lesions of these two patients were not verrucous carcinoma rather than RRP [47]. When these two patients would be excluded from this review there would have been three patients with dysplasia on a total of 186 treated patients. This corresponds to a percentage of 1.6. Since it is not definitely sure that the verrucous carcinoma was present before the start of therapy with cidofovir the two patients have been included in our study. The follow-up period of the included studies varied. It cannot be ruled out that there are patients in which dysplasia has occurred after the follow-up period.
In two of the five patients with dysplasia the HPV-subtype has been identified. In these two patients HPV-6 and HPV-11 were found. The high-risk types HPV-16 and 18 were not present. This is not unusual, as becomes clear from the various articles that have been published in which only the HPV-subtypes 6 and 11 were found in patients with RRP that had undergone malignant degeneration [8, 16, 27, 31, 34, 41, 44, 49].
The known cumulative dosages in the patients in which dysplasia has occurred (30 and 83.5 mg) do not differ from the cumulative dosages that have been administered in other studies. In one study, the mean cumulative dose is even 348 mg [9]. A cumulative effect on the development of dysplasia is, therefore, unlikely.
The safety of intralesional injections with cidofovir has also been evaluated in animals [10, 24, 52]. Chhetri et al. [10] used a canine model to investigate the effects of cidofovir. A 6-month period of intralaryngeal injection of cidofovir induced dose-dependent localized toxic injury to muscle and induced vocal fold atrophy and scarring. These effects appeared to be irreversible in the animals that received a dose higher than 20 mg of cidofovir. There were no clinically significant changes in leukocyte count, renal parameters, electrolytes, or liver function tests. No evidence of dysplasia, metaplasia, or carcinoma of the vocal folds was observed.
Spiegel et al. [52] evaluated the local effects of cidofovir injection on cartilage in rabbits during 6 weeks. A total of 96 sites were injected with cidofovir in concentrations of 0, 5, 25, and 75 mg. One third of the injection sites showed a gross change in appearance (mild to moderate crusting, some with erythema), whereas two thirds showed no visible change at any time during the 6 weeks. Although there was a statistical likelihood for increased local change after cidofovir injection, there was no correlation of severity with injected dose.
In a 26-week intravenous toxicology study in which rats received 0.6, 3, or 15 mg/kg cidofovir once weekly, a significant increase in mammary adenocarcinomas in female rats was observed [24]. Furthermore, a significant incidence of Zymbal’s gland carcinomas in male and female rats was seen at the high dose but not at the lower two doses. Cidofovir caused no tumours in a 1-year monkey toxicity study. In this study, cynomolgus monkeys received intravenous cidofovir, alone and in conjunction with concomitant oral probenecid, intravenously once weekly for 52 weeks. They received doses resulting in exposures of approximately 0.7 times the human systemic exposure at the recommended dose of cidofovir.
The findings in rats raised the concern about the potential carcinogenicity of cidofovir. It is noteworthy, however, that the development of mammary adenocarcinoma in rats is a frequent occurrence in rat pharmacologic studies [19].
In the four cases in which the cidofovir was administered intravenously the RRP had an aggressive course with pulmonal extension. Pulmonary involvement of RRP can lead to respiratory insufficiency and even death [45]. Few side-effects have occurred in the four cases in which the cidofovir was administered intravenously [2, 17, 18, 53]. In one case partial alopecia and leukopenia occurred after 34 injections with cidofovir in a period of 16 months [17]. Nefrotoxicity was not noted in the four described cases. Therefore, pulmonary involvement of RRP seems a justified indication for the intravenous administration of cidofovir. The administration of oral probenecid and intravenous hydration before and after each cidofovir dose remains important.
Because there are no adequate and well-controlled studies in pregnant women and cidofovir has shown to be teratogenic and embryotoxic in animal studies, this medicine is classified by the FDA as a pregnancy category C drug [25]. The FDA recommends using category C drugs only during pregnancy if clearly needed [23]. Because RRP can also be controlled with repeated microsurgery the use of cidofovir during pregnancy can be avoided. It is noteworthy, however, that the recommendations of the FDA for cidofovir during pregnancy are based on the systemic administration of cidofovir.
In a combined in vitro and in vivo study on the teratogenicity of cidofovir a cytostatic effect on eukaryotic cells and a generalized embryolethal effect in pregnant rats was found [7]. The cidofovir was administered to the rats in a relatively high concentration of 20–100 mg/kg. For the intralesional administration of cidofovir concentrations below 3 mg/kg are being used. As noted above the plasma cidofovir concentrations after intralesional administration are remarkably lower than those achieved with the systemic administration of cidofovir [38]. Furthermore, no systemic side-effects have been reported during treatment with intralesional cidofovir. Therefore, the question rises if teratogenic effects are indeed to be expected during the use of intralesional cidofovir. More studies in the future are necessary to answer this question. Until that time it remains recommended to be reserved with the use of intralesional cidofovir during pregnancy.
Conclusion
Based on the published literature it can be concluded that the therapy with intralesional cidofovir for patients with RRP does not increase the risk of laryngeal dysplasia. Systemic side-effects have not been reported in patients who have received intralesional cidofovir. Treatment with intralesional cidofovir seems to be safe provided that the regulations for this therapy are being followed. These regulations comprehend that the intralesionally injected dose of cidofovir in adults should be below 3 mg/kg. During pregnancy, it is recommended to be reserved with the use of intralesional cidofovir. Pulmonary involvement of RRP seems a justified indication for the intravenous administration of cidofovir.
The treatment with cidofovir should be given in a tertiary care hospital because of the low incidence of RRP. Furthermore, it remains important to monitor the potential side-effects of cidofovir and always histologically analyze the biopsies taken from patients who received treatment with cidofovir. The publication of encountered side-effects and occurrence of dysplasia during treatment with intralesionally applied cidofovir is important for the evaluation of the safety of cidofovir in the future.
References
Akst LM, Lee W, Discolo C, Knott D, Younes A, Koltai PJ (2003) Stepped-dose protocol of cidofovir therapy in recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg 129:841–846
Armbruster C, Kreuzer A, Vorbach H, Huber M, Armbruster C (2001) Successful treatment of severe respiratory papillomatosis with intravenous cidofovir and interferon alpha-2b. Eur Respir J 17:830–831
Askew J, Black R (2005) Intralesional cidofovir use in recurrent respiratory papillomatosis of childhood. Aust J Otolaryng 8:26–29
Avelino M, Gutzman R, Fujita R, Pignatari S, Weckx L, Pontes P (2004) Cidofovir effects on recurrent laryngeal papillomatosis in children: preliminary report. Rev Bras Otorrinolaringol 70:734–738
Bauman NM, Smith RJ (1996) Recurrent respiratory papillomatosis. Pediatr Clin North Am 43:1385–1401
Bielamowicz S, Villagomez V, Stager SV, Wilson WR (2002) Intralesional cidofovir therapy for laryngeal papilloma in an adult cohort. Laryngoscope 112:696–699
Bila V, Otova B, Jelinek R, Sladka M, Mejsnarova B, Holy A, Kren V (1993) Antimitotic and teratogenic effects of acyclic nucleotide analogues 1-(S)-(3-hydroxy-2-phosphonomethoxyethyl)cytosine (HPMPC) and 9-(2-phosphonomethoxyethyl) adenine (PMEA). Folia Biol (Praha) 39:150–161
Byrne JC, Tsao MS, Fraser RS, Howley PM (1987) Human papillomavirus-11 DNA in a patient with chronic laryngotracheobronchial papillomatosis and metastatic squamous-cell carcinoma of the lung. N Engl J Med 317:873–878
Chhetri DK, Blumin JH, Shapiro NL, Berke GS (2002) Office-based treatment of laryngeal papillomatosis with percutaneous injection of cidofovir. Otolaryngol Head Neck Surg 126:642–648
Chhetri DK, Jahan-Parwar B, Hart SD, Bhuta SM, Berke GS, Shapiro NL (2003) Local and systemic effects of intralaryngeal injection of cidofovir in a canine model. Laryngoscope 113:1922–1926
Chhetri DK, Shapiro NL (2003) A scheduled protocol for the treatment of juvenile recurrent respiratory papillomatosis with intralesional cidofovir. Arch Otolaryngol Head Neck Surg 129:1081–1085
Chung BJ, Akst LM, Koltai PJ (2006) 3.5-Year follow-up of intralesional cidofovir protocol for pediatric recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol 70:1911–1917
Co J, Woo P (2004) Serial office-based intralesional injection of cidofovir in adult-onset recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 113:859–862
Coulombeau B, Nusa NA, Ceruse P, Froehlich P (2002) [Anti-viral injectable treatment (cidofovir) in laryngeal papillomatosis]. Rev Laryngol Otol Rhinol (Bord) 123:315–320
Cundy KC (1999) Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet 36:127–143
Dallimore NS (1985) Squamous bronchial carcinoma arising in a case of multiple juvenile papillomatosis. Thorax 40:797–798
Dancey DR, Chamberlain DW, Krajden M, Palefsky J, Alberti PW, Downey GP (2000) Successful treatment of juvenile laryngeal papillomatosis-related multicystic lung disease with cidofovir: case report and review of the literature. Chest 118:1210–1214
de Bilderling G, Bodart E, Lawson G, Tuerlinckx D, Remacle M, Naesens L, De Clercq E, Snoeck R (2005) Successful use of intralesional and intravenous cidofovir in association with indole-3-carbinol in an 8-year-old girl with pulmonary papillomatosis. J Med Virol 75:332–335
Derkay C (2005) Cidofovir for recurrent respiratory papillomatosis (RRP): a re-assessment of risks. Int J Pediatr Otorhinolaryngol 69:1465–1467
Dikkers FG (2006) Treatment of recurrent respiratory papillomatosis with microsurgery in combination with intralesional cidofovir-a prospective study. Eur Arch Otorhinolaryngol 263:440–443
El Aatmani M, Steinmetz A, Debry D, Levêque D, Koffel J-C, Beretz L (2002) À propos d’un cas de traitement de la papillomatose laryngée récidivante par injections intralésionnelles de cidofovir (Vistide). J Pharm Clin 21:287–290
El Hakim H, Waddell AN, Crysdale WS (2002) Observations on the early results of treatment of recurrent respiratory papillomatosis using cidofovir. J Otolaryngol 31:333–335
Food and Drug Administration. http://www.fda.gov/cder/handbook/categc.htm
Food and Drug Administration. 1996 http://www.fda.gov/cder/foi/adcomm/96/avdac_joint_031496_summmin_ac.pdf
Gilead Sciences. Inc. 2000 Vistide (cidofovir) package insert. Foster City, CA
Giles BL, Seifert B (2006) CR12/339–Nebulized cidofovir for recurrent respiratory papillomatosis: a case report. Paediatr Respir Rev 7(Suppl 1):S330
Guillou L, Sahli R, Chaubert P, Monnier P, Cuttat JF, Costa J (1991) Squamous cell carcinoma of the lung in a nonsmoking, nonirradiated patient with juvenile laryngotracheal papillomatosis. Evidence of human papillomavirus-11 DNA in both carcinoma and papillomas. Am J Surg Pathol 15:891–898
Hoffman VF, Skiest DJ (2000) Therapeutic developments in cytomegalovirus retinitis. Expert Opin Investig Drugs 9:207–220
Kimberlin DW (2004) Current status of antiviral therapy for juvenile-onset recurrent respiratory papillomatosis. Antiviral Res 63:141–151
Lee AS, Rosen CA (2004) Efficacy of cidofovir injection for the treatment of recurrent respiratory papillomatosis. J Voice 18:551–556
Lindeberg H, Syrjanen S, Karja J, Syrjanen K (1989) Human papillomavirus type 11 DNA in squamous cell carcinomas and pre-existing multiple laryngeal papillomas. Acta Otolaryngol 107:141–149
Mandell DL, Arjmand EM, Kay DJ, Casselbrant ML, Rosen CA (2004) Intralesional cidofovir for pediatric recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 130:1319–1323
Milczuk HA (2003) Intralesional cidofovir for the treatment of severe juvenile recurrent respiratory papillomatosis: long-term results in 4 children. Otolaryngol Head Neck Surg 128:788–794
Moore CE, Wiatrak BJ, McClatchey KD, Koopmann CF, Thomas GR, Bradford CR, Carey TE (1999) High-risk human papillomavirus types and squamous cell carcinoma in patients with respiratory papillomas. Otolaryngol Head Neck Surg 120:698–705
Naiman AN, Abedipour D, Ayari S, Fresnel E, Coulombeau B, Bour JB, Froehlich P (2006) Natural history of adult-onset laryngeal papillomatosis following multiple cidofovir injections. Ann Otol Rhinol Laryngol 115:175–181
Naiman AN, Ayari S, Nicollas R, Landry G, Coulombeau B, Froehlich P (2006) Intermediate-term and long-term results after treatment by cidofovir and excision in juvenile laryngeal papillomatosis. Ann Otol Rhinol Laryngol 115:667–672
Naiman AN, Ceruse P, Coulombeau B, Froehlich P (2003) Intralesional cidofovir and surgical excision for laryngeal papillomatosis. Laryngoscope 113:2174–2181
Naiman AN, Roger G, Gagnieu MC, Bordenave J, Mathaut S, Ayari S, Nicollas R, Bour JB, Garabedian N, Froehlich P (2004) Cidofovir plasma assays after local injection in respiratory papillomatosis. Laryngoscope 114:1151–1156
Neumann K, Pudszuhn A, Welzel C, Bartel-Friedrich S, Passmann M (2003) [Intralesional cidofovir injections for recurrent laryngeal papillomatosis: first results]. Laryngorhinootologie 82:700–706
Palomar AV, Palomar GV, Soteras OJ, Ruiz GA (2005) [Cidofovir activity in infantile recurrent respiratory papillomatosis]. Acta Otorrinolaringol Esp 56:22–24
Petersen BL, Buchwald C, Gerstoft J, Bretlau P, Lindeberg H (1998) An aggressive and invasive growth of juvenile papillomas involving the total respiratory tract. J Laryngol Otol 112:1101–1104
Peyton SW, Wiatrak B (2004) Is cidofovir a useful adjunctive therapy for recurrent respiratory papillomatosis in children? Int J Pediatr Otorhinolaryngol 68:413–418
Pontes P, Avelino M, Pignatari S, Weckx LL (2006) Effect of local application of cidofovir on the control of recurrences in recurrent laryngeal papillomatosis. Otolaryngol Head Neck Surg 135:22–27
Pou AM, Rimell FL, Jordan JA, Shoemaker DL, Johnson JT, Barua P, Post JC, Ehrlich GD (1995) Adult respiratory papillomatosis: human papillomavirus type and viral coinfections as predictors of prognosis. Ann Otol Rhinol Laryngol 104:758–762
Pransky SM, Albright JT, Magit AE (2003) Long-term follow-up of pediatric recurrent respiratory papillomatosis managed with intralesional cidofovir. Laryngoscope 113:1583–1587
Pransky SM, Brewster DF, Magit AE, Kearns DB (2000) Clinical update on 10 children treated with intralesional cidofovir injections for severe recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 126:1239–1243
Pransky SM, Magit AE, Kearns DB, Kang DR, Duncan NO (1999) Intralesional cidofovir for recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg 125:1143–1148
Pudszuhn A, Welzel C, Bloching M, Neumann K (2007) Intralesional Cidofovir application in recurrent laryngeal papillomatosis. Eur Arch Otorhinolaryngol 264:63–70
Rady PL, Schnadig VJ, Weiss RL, Hughes TK, Tyring SK (1998) Malignant transformation of recurrent respiratory papillomatosis associated with integrated human papillomavirus type 11 DNA and mutation of p53. Laryngoscope 108:735–740
Sheahan P, Sexton S, Russell JD (2006) Is intralesional cidofovir worthwhile in juvenile recurrent respiratory papillomatosis? J Laryngol Otol 120:561–565
Snoeck R, Wellens W, Desloovere C, Van Ranst M, Naesens L, De Clercq E, Feenstra L (1998) Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine]. J Med Virol 54:219–225
Spiegel JH, Andrus JG, Stefanato CM, Heeren T (2005) Histopathologic effects of cidofovir on cartilage. Otolaryngol Head Neck Surg 133:666–671
Van Valckenborgh I, Wellens W, De Boeck K, Snoeck R, De Clercq E, Feenstra L (2001) Systemic cidofovir in papillomatosis. Clin Infect Dis 32:E62–E64
Wachsman M, Petty BG, Cundy KC, Jaffe HS, Fisher PE, Pastelak A, Lietman PS (1996) Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res 29:153–161
Wemer RD, Lee JH, Hoffman HT, Robinson RA, Smith RJ (2005) Case of progressive dysplasia concomitant with intralesional cidofovir administration for recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 114:836–839
Wilson WR, Hashemiyoon R, Hawrych A (2000) Intralesional cidofovir for recurrent laryngeal papillomas: preliminary report. Ear Nose Throat J 79:236–238, 240
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Broekema, F.I., Dikkers, F.G. Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol 265, 871–879 (2008). https://doi.org/10.1007/s00405-008-0658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-008-0658-0