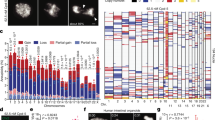
Abstract
Chromosome engineering has allowed the generation of an extensive and well-defined series of linear human X centromere-based minichromosomes, which has been used to investigate the influence of size and structure on chromosome segregation in vertebrate cells. A clear relationship between overall chromosome size and mitotic stability was detected, with decreasing size associated with increasing loss rates. In chicken DT40, the lower size limit for prolonged mitotic stability is approximately 550 kb: at 450 kb, there was a dramatic increase in chromosome loss, while structures of approximately 200 kb could not be recovered. In human HT1080 cells, the size threshold for mitotic stability is approximately 1.6 Mb. Minichromosomes of 0.55–1.0 Mb can be recovered, but display high loss rates. However, all minichromosomes examined exhibited more segregation errors than normal chromosomes in HT1080 cells. This error rate increases with decreased size and correlates with reduced levels of CENP-A and Aurora B. In mouse LA-9 and Indian muntjac FM7 cells, the size requirements for mitotic stability are much greater. In mouse, a human 2.7-Mb minichromosome is rarely able to propagate a kinetochore and behaves acentrically. In Indian muntjac, CENP-C associates with the human minichromosome, but the mitotic apparatus appears unable to handle its segregation.







Similar content being viewed by others
References
Alazami A, Mejia JE, Larin Monaco Z (2004) Human artificial chromosomes containing chromosome 17 alphoid DNA maintain an active centromere in murine cells but are not stable. Genomics 83:844–851
Andrews PD, Ovechkina Y, Morrice N et al (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6:253–268
Araki K, Araki M, Yamamura K-i (1997) Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res 25:868–872
Basu J, Willard HF (2005) Artificial and engineered chromosomes: non-integrating vectors for gene therapy. Trends Mol Med 11:251–258
Burns EM, Christopoulou L, Corish P, Tyler-Smith C (1999) Quantitative measurement of mammalian chromosome mitotic loss rates using the green fluorescent protein. J Cell Sci 112:2705–2714
Conese M, Auriche C, Ascenzioni F (2004) Gene therapy progress and prospects: episomally maintained self-replicating systems. Gene Ther 11:1735–1741
Corish P, Tyler-Smith C (1999) Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng 12:1035–1040
Dieken ES, Fournier REK (1996) Homologous modification of human chromosomal genes in chicken B-cell × human microcell hybrids. Methods 9(1):56–63
Dieken ES, Epner EM, Fiering S, Fournier REK, Groudine M (1996) Efficient modification of human chromosomal alleles using recombination-proficient chicken/human microcell hybrids. Nat Genet 12:174–182
Dunleavy E, Pidoux A, Allshire RC (2005) Centromeric chromatin makes its mark. Trends Biochem Sci 30:172–175
Farr CJ, Stevanovic M, Thomson EJ, Goodfellow PN, Cooke HJ (1992) Telomere-associated chromosome fragmentation: applications in genome manipulation and analysis. Nat Genet 2:275–282
Farr CJ, Bayne RAL, Kipling D, Mills W, Critcher R, Cooke HJ (1995) Generation of a human X-derived minichromosome using telomere-associated chromosome fragmentation. EMBO J 14:5444–5454
Featherstone T, Huxley C (1993) Extrachromosomal maintenance and amplification of yeast artificial chromosome DNA in mouse cells. Genomics 17:267–278
Feinberg AP, Vogelstein B (1984) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137:266–267
Fukagawa T (2004) Centromere DNA, proteins and kinetochore assembly in vertebrate cells. Chromosome Res 12:557–567
Fukagawa T, Pendon C, Morris J, Brown W (1999) CENP-C is necessary but not sufficient to induce formation of a functional centromere. EMBO J 18:4196–4209
Fukagawa T, Mikami Y, Nishihashi A et al (2001) CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J 20:4603–4617
Fukagawa T, Nogami M, Yoshikawa M et al (2004) Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol 6:784–791
Grimes BR, Warburton PE, Farr CJ (2002) Chromosome engineering: prospects for gene therapy. Gene Ther 9:713–718
Guenatri M, Bailly D, Maison C, Almouzni G (2004) Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol 166:493–505
Hauf S, Cole RW, LaTerra S et al (2003) The small molecule hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161:281–294
He D, Brinkley BR (1996) Structure and dynamic organization of centromeres/prekinetochores in the nucleus of mammalian cells. J Cell Sci 109:2693–2704
Howman AE, Fowler KJ, Newson AJ et al (2000) Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci U S A 97:1148–1153
Ikeno M, Grimes B, Okazaki T et al (1998) Construction of YAC-based mammalian artificial chromosomes. Nat Biotechnol 16:431–439
Irvine DV, Amor DJ, Perry J et al (2004) Chromosome size and origin as determinants of the level of CENP-A incorporation into human chromosomes. Chromosome Res 12:805–815
Kazuki Y, Shinohara T, Tomizuka K et al (2001) Germline transmission of a transferred human chromosome 21 fragment in transchromosomal mice. J Hum Genet 46:600–603
Kuroiwa Y, Tomizuka K, Shinohara T et al (2000) Manipulation of human minichromosomes to carry greater than megabase-sized chromosome inserts. Nature Biotechnol 18:1086–1090
Lee JY, Koi M, Stanbridge EJ, Oshimura M, Kumamoto AT, Feinberg AP (1994) Simple purification of human chromosomes to homogeneity using muntjac hybrid cells. Nat Genet 7:29–33
Loupart M-L, Shen MH, Smith A (1998) Differential stability of a human mini-chromosome in mouse cell lines. Chromosoma 107:255–259
Mellone BG, Allshire RC (2003) Stretching it: putting the CEN(P-A) in centromere. Curr Opin Genet Dev 13:191–198
Mills W, Critcher R, Lee C, Farr CJ (1999) Generation of an approximately 2.4 Mb human X centromere-based minichromosome by targeted telomere-associated chromosome fragmentation in DT40. Hum Mol Genet 8:751–761
Murray AW, Schultes NP, Szostak JW (1986) Chromosome length controls mitotic segregation in yeast. Cell 45:529–536
Niwa H, Yamamura K-i, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199
Pichugin AM, Galkina SA, Potekhin AA, Punina EO, Rautian MS, Rodionov AV (2001) Determination of the minimum size of Gallus gallus domesticus chicken microchromosome by a pulse electrophoresis method. Genetika 37:657–660
Regnier V, Novelli J, Fukagawa T, Vagnarelli P, Brown W (2003) Characterization of chicken CENP-A and comparative sequence analysis of vertebrate centromere-specific histone H3-like proteins. Gene 316:39–46
Rudd MK, Mays RW, Schwatz S, Willard HF (2003) Human artificial chromosomes with alpha satellite-based de novo centromeres show increased frequency of non-disjunction and anaphase lag. Mol Cell Biol 23:7689–7697
Saitoh H, Tomkiel J, Cooke CA et al (1992) CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70:115–125
Sauer B, Henderson N (1990) Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol 2:441–449
Schafer AJ, Farr CJ (1998) Somatic cell hybrid approaches to mapping. In: Spurr NK, Young BD, Bryant SP (eds) The ICRF handbook of genome analysis. Blackwell, Oxford, pp 321–365
Schueler MG, Higgins AW, Rudd MK, Gustashaw K, Willard HF (2001) Genomic and genetic definition of a functional human centromere. Science 294:109–115
Shen MH, Mee PJ, Nichols J et al (2000) A structurally defined mini-chromosome vector for the mouse germ line. Curr Biol 10:31–34
Shen MH, Yang JW, Pendon C, Brown WR (2001) The accuracy of segregation of human mini-chromosomes varies in different vertebrate cell lines, correlates with the extent of centromere formation and provides evidence for a trans-acting centromere maintenance activity. Chromosoma 109:524–535
Shinohara T, Tomizuka K, Takehara S et al (2000) Stability of transferred human chromosome fragments in cultured cells and in mice. Chromosome Res 8:713–725
Spence JM, Critcher R, Ebersole TA et al (2002) Co-localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha-satellite array. EMBO J 21:5269–5280
Spence JM, Fournier REK, Oshimura M, Regnier V, Farr CJ (2005) Topoisomerase II cleavage activity within the D11Z1 and DXZ1 alpha-satellite arrays. Chromosome Res 13(6):637–648
Sullivan BA, Karpen GH (2004) Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol 11:1076–1083
Sullivan BA, Warburton PE (1999) Studying the progression of vertebrate chromosomes through mitosis by immunofluorescence and FISH. In: Bickmore WA (ed) Chromosome structural analysis: a practical approach. Oxford University Press, Oxford, pp 82–101
Taylor SS, Larin Z, Tyler-Smith C (1996) Analysis of extrachromosomal structures containing human centromeric alphoid satellite DNA sequences in mouse cells. Chromosoma 105:70–81
Tomizuka K, Yoshida H, Uejima H et al (1997) Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat Genet 16:133–143
Tomizuka K, Shinohara T, Yoshida H et al (2000) Double trans-chromosomic mice: maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc Natl Acad Sci U S A 97:722–727
Valdivia MM, Figueroa J, Iglesias C, Ortiz M (1998) A novel centromere monospecific serum to a human autoepitope on the histone H3-like protein CENP-A. FEBS Lett 422:5–9
Voet T, Vermeesch J, Carens A et al (2001) Efficient male and female germline transmission of a human chromosomal vector in mice. Genome Res 11:124–136
Voet T, Schoenmakers E, Carpentier S, Labaere C, Marynen P (2003) Controlled transgene dosage and PAC-mediated transgenesis in mice using a chromosomal vector. Genomics 82:596–605
Wong LH, Saffery R, Anderson MA, Earle E, Quach JM, Stafford AJ, Fowler KJ, Choo KH (2005) Analysis of mitotic and expression properties of human neocentromere-based transchromosomes in mice. J Biol Chem 280(5):3954–3962
Yang J, Pendon C, Yang J, Haywood N, Chand A, Brown WRA (2000) Human mini-chromosomes with minimal centromeres. Hum Mol Genet 9:1891–1902
Zou Y, Yi X, Wright WE, Shay JW (2002) Human telomerase can immortalize Indian muntjac cells. Exp Cell Res 281:63–76
Acknowledgements
We are grateful to the following for their generous gifts of antibodies: Bill Earnshaw (anti-human CENP-C); Manuel Valdivia (anti-human CENP-A); Paul Kalitsis and Andy Choo (anti-mouse CENP-C and -A); Vicianne Regnier (anti-chicken CENP-A) and Tats Fukagawa (anti-chicken CENP-H and -C). For cell lines, we thank Andrew Feinberg (FM7), Jerry Shay (IndianMuntert) and Bill Colledge (CCB mouse ES cells). We thank Chris Tyler-Smith for the pGFP-PEST:IRES:Zeo reporter cassette, and Andy Jessop and Nigel Miller for their help with flow cytometry. This work was funded by project grants from the Biotechnology and Biological Sciences Research Council (BBSRC 8/GTH12583/4), the EC Framework 5 (QLK3-CT-2002-02119) and, in its early stages, by a Medical Research Council Senior Research fellowship to CJF. We thank the BBSRC for the flow cytometer research equipment initiative grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Masumoto
Rights and permissions
About this article
Cite this article
Spence, J.M., Mills, W., Mann, K. et al. Increased missegregation and chromosome loss with decreasing chromosome size in vertebrate cells. Chromosoma 115, 60–74 (2006). https://doi.org/10.1007/s00412-005-0032-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-005-0032-6