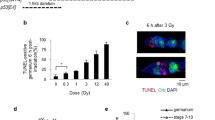
Abstract
In Drosophila, males absent on the first (MOF) acetylates histone H4 at lysine 16 (H4K16ac). This acetylation mark is highly enriched on the male X chromosome and is required for dosage compensation in Drosophila but not utilized for such in mammals. Recently, we and others reported that mammalian MOF, through H4K16ac, has a critical role at multiple stages in the DNA damage response (DDR) and double-strand break repair pathways. The goal of this study was to test whether mof is similarly required for the response to ionizing radiation (IR) in Drosophila. We report that Drosophila mof mutations in males and females, as well as mof knockdown in SL-2 cells, reduce post-irradiation survival. MOF depletion in SL-2 cells also results in an elevated frequency of metaphases with chromosomal aberrations, suggesting that MOF is involved in DDR. Mutation in Drosophila mof also results in a defective mitotic checkpoint, enhanced apoptosis, and a defective p53 response post-irradiation. In addition, IR exposure enhanced H4K16ac levels in Drosophila as it also does in mammals. These results are the first to demonstrate a requirement for MOF in the whole animal IR response and suggest that the role of MOF in the response to IR is conserved between Drosophila and mammals.










Similar content being viewed by others
References
Akhtar A, Becker PB (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell 5:367–375
Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM (2004) Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 24:1219–1231
Fischle W, Wang Y, Allis CD (2003) Histone and chromatin cross-talk. Curr Opin Cell Biol 15:172–183
Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK (2005) Involvement of human MOF in ATM function. Mol Cell Biol 25:5292–5305
Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK et al (2008) The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol 28:397–409
Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi JC (1997) mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J 16:2054–2060
Hoeijmakers JH (2001) DNA repair mechanisms. Maturitas 38:17–22, discussion 22–13
Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK (2004) Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol 24:899–911
Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R et al (2007) Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res 67:3010–3017
Jaklevic BR, Su TT (2004) Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr Biol 14:23–32
Kageyama Y, Mengus G, Gilfillan G, Kennedy HG, Stuckenholz C, Kelley RL, Becker PB, Kuroda MI (2001) Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J 20:2236–2245
Kastan MB (2008) DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol Cancer Res 6:517–524
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ Jr (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587–597
Kind J, Vaquerizas JM, Gebhardt P, Gentzel M, Luscombe NM, Bertone P, Akhtar A (2008) Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell 133:813–828
Kumar R, Hunt CR, Gupta A, Nannepaga S, Pandita RK, Shay JW, Bachoo R, Ludwig T, Burns DK, Pandita TK (2011) Purkinje cell-specific males absent on the first (mMof) gene deletion results in an ataxia-telangiectasia-like neurological phenotype and backward walking in mice. Proc Natl Acad Sci USA 108:3636–3641
Legube G, Trouche D (2003) Regulating histone acetyltransferases and deacetylases. EMBO Rep 4:944–947
Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, Min J, Dou Y (2010) MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Mol Cell Biol 30:5335–5347
Misri S, Pandita S, Kumar R, Pandita TK (2008) Telomeres, histone code, and DNA damage response. Cytogenet Genome Res 122:297–307
Neal KC, Pannuti A, Smith ER, Lucchesi JC (2000) A new human member of the MYST family of histone acetyl transferases with high sequence similarity to Drosophila MOF. Biochim Biophys Acta 1490:170–174
Otten AD, Tapscott SJ (1995) Triplet repeat expansion in myotonic dystrophy alters the adjacent chromatin structure. Proc Natl Acad Sci USA 92:5465–5469
Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, Elgin SC (2004) Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303:669–672
Pandita TK (2001) The role of ATM in telomere structure and function. Radiat Res 156:642–647
Pandita TK (2002) ATM function and telomere stability. Oncogene 21:611–618
Pandita TK (2003) A multifaceted role for ATM in genome maintenance. Expert Rev Mol Med 5:1–21
Pandita TK (2006) Role of mammalian Rad9 in genomic stability and ionizing radiation response. Cell Cycle 5:1289–1291
Pandita TK, Geard CR (1996) Chromosome aberrations in human fibroblasts induced by monoenergetic neutrons. I. Relative biological effectiveness. Radiat Res 145:730–739
Pandita TK, Hittelman WN (1992a) The contribution of DNA and chromosome repair deficiencies to the radiosensitivity of ataxia-telangiectasia. Radiat Res 131:214–223
Pandita TK, Hittelman WN (1992b) Initial chromosome damage but not DNA damage is greater in ataxia telangiectasia cells. Radiat Res 130:94–103
Pandita TK, Richardson C (2009) Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res 37:1363–1377
Pandita TK, Lieberman HB, Lim DS, Dhar S, Zheng W, Taya Y, Kastan MB (2000) Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene 19:1386–1391
Pandita RK, Sharma GG, Laszlo A, Hopkins KM, Davey S, Chakhparonian M, Gupta A, Wellinger RJ, Zhang J, Powell SN et al (2006) Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol Cell Biol 26:1850–1864
Pruss D, Bushman FD, Wolffe AP (1994) Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. Proc Natl Acad Sci USA 91:5913–5917
Raja SJ, Charapitsa I, Conrad T, Vaquerizas JM, Gebhardt P, Holz H, Kadlec J, Fraterman S, Luscombe NM, Akhtar A (2010) The nonspecific lethal complex is a transcriptional regulator in Drosophila. Mol Cell 38:827–841
Richardson C, Horikoshi N, Pandita TK (2004) The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst) 3:1149–1164
Scott SP, Pandita TK (2006) The cellular control of DNA double-strand breaks. J Cell Biochem 99:1463–1475
Sharma GG, Hwang KK, Pandita RK, Gupta A, Dhar S, Parenteau J, Agarwal M, Worman HJ, Wellinger RJ, Pandita TK (2003) Human heterochromatin protein 1 isoforms HP1(Hsalpha) and HP1(Hsbeta) interfere with hTERT−telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol Cell Biol 23:8363–8376
Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ et al (2010) MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol 30:3582–3595
Smith ER, Pannuti A, Gu W, Steurnagel A, Cook RG, Allis CD, Lucchesi JC (2000) The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol Cell Biol 20:312–318
Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC (2005) A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol 25:9175–9188
Song YH, Mirey G, Betson M, Haber DA, Settleman J (2004) The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr Biol 14:1354–1359
Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A (2005) hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol 25:6798–6810
van Attikum H, Gasser SM (2005) The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol 6:757–765
van Attikum H, Gasser SM (2009) Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol 19:207–217
Wallrath LL, Lu Q, Granok H, Elgin SC (1994) Architectural variations of inducible eukaryotic promoters: preset and remodeling chromatin structures. Bioessays 16:165–170
Wolff T, Ready DF (1991a) The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development 113:841–850
Wolff T, Ready DF (1991b) Cell death in normal and rough eye mutants of Drosophila. Development 113:825–839
Worby CA, Simonson-Leff N, Dixon JE (2001) RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci STKE 2001(95):pl1
Zhang Y, Malone JH, Powell SK, Periwal V, Spana E, MacAlpine DM, Oliver B (2010) Expression in aneuploid Drosophila S2 cells. PLoS Biol 8(2):e1000320. doi:10.1371/journal.pbio.1000320
Acknowledgments
Thanks are due to Edwin Smith and Peter Becker for providing regents and valuable suggestions and to Fan Zhang with help in irradiating mof 3 /mof 6 flies. We also thank members of Pandita laboratory for helpful discussions and suggestions.
Funding
This work was supported by NIH NCI grants R01CA123232, R01CA129537, RO1CA154320, and R13CA130756 (TKP); NSF grants MCB 0131414 and MCB 0615831 (JCE); NIGMS grant RO115691 (JCL); and Senior Wellcome Trust fellowship grants, GRA070065MA (UB) and (GRA076395AIA) (MPB).
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhadra, M.P., Horikoshi, N., Pushpavallipvalli, S.N. et al. The role of MOF in the ionizing radiation response is conserved in Drosophila melanogaster . Chromosoma 121, 79–90 (2012). https://doi.org/10.1007/s00412-011-0344-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-011-0344-7