Abstract
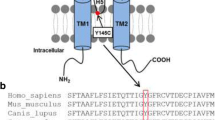
Barttin, a gene product of BSND, was identified as a fourth gene responsible for Bartter syndrome. The co-expression of barttin with CLC-K chloride channels has been demonstrated to dramatically induce the expression of CLC-K current. However, it remains unknown how barttin interacts with CLC-K channels in mammalian cells and how the mutations of barttin lead to Bartter syndrome. In an attempt to clarify the effect of barttin expression on CLC-K2 cellular localization, we examined the expression of the CLC-K2 chloride channel and barttin, solely and in combination, in transient and stable expression systems in mammalian cells. In addition, we generated several stable cell lines expressing mutant barttins to clarify the consequence of the previously reported barttin mutations in Bartter syndrome. In immunocytochemistry, CLC-K2 was retained in the Golgi in the absence of barttin expression, but delivered to the plasma membrane when barttin was present. Barttin was coprecipitated with CLC-K2, suggesting a protein–protein interaction. Disease-causing mutant barttins, especially R8L, were retained intracellularly, but their binding ability to CLC-K2 was preserved. This led to a retention of CLC-K2 in intracellular organelles with barttin, and a loss of plasma membrane localization. The stability of the CLC-K2 protein was also markedly increased by coexpression with barttin. These results clarified that barttin determined cellular localization of CLC-K2 by protein–protein interaction. Thus, the mislocalization of CLC-K2 was identified as the molecular pathogenesis of Bartter syndrome by mutant barttins.







Similar content being viewed by others
References
Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F, Sasaki S (1994) Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J Biol Chem 269:17677–17683
Akizuki N, Uchida S, Sasaki S, Marumo F (2001) Impaired solute accumulation in inner medulla of Clcnk1−/− mice kidney. Am J Physiol Renal Physiol 280:F79–F87
Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29:310–314
Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415:287–294
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414:558–561
Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ (1994) Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A 91:6943–6947
Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F (2001a) Developmental expression of CLC-K1 in the postnatal rat kidney. Histochem Cell Biol 116:49–56
Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F (2001b) Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12:1327–1334
Kobayashi K, Uchida S, Okamura HO, Marumo F, Sasaki S (2002) Human CLC-KB gene promoter drives the EGFP expression in the specific distal nephron segments and inner ear. J Am Soc Nephrol 13:1992–1998
Matsumura Y, Uchida S, Kondo Y, Miyazaki H, Ko SB, Hayama A, Morimoto T, Liu W, Arisawa M, Sasaki S, Marumo F (1999) Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 21:95–98
Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC (1996) Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 15:2381–2387
Schwake M, Friedrich T, Jentsch TJ (2001) An internalization signal in ClC-5, an endosomal Cl-channel mutated in Dent's disease. J Biol Chem 276:12049–12054
Simon DB, Lifton RP (1996) The molecular basis of inherited hypokalemic alkalosis: Bartter's and Gitelman's syndromes. Am J Physiol 271:F961–F966
Tamarappoo BK, Yang B, Verkman AS (1999) Misfolding of mutant aquaporin-2 water channels in nephrogenic diabetes insipidus. J Biol Chem 274:34825–34831
Uchida S, Sasaki S, Furukawa T, Hiraoka M, Imai T, Hirata Y, Marumo F (1993) Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla. J Biol Chem 268:3821–3824
Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F (1995) Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J Clin Invest 95:104–113
Waldegger S, Jentsch TJ (2000) Functional and structural analysis of ClC-K chloride channels involved in renal disease. J Biol Chem 275:24527–24533
Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW (2002) Barttin increases surface expression and changes current properties of ClC-K channels. Pflugers Arch 444:411–418
Welsh MJ, Smith AE (1993) Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251–1254
Acknowledgements
This study was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Salt Science Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayama, A., Rai, T., Sasaki, S. et al. Molecular mechanisms of Bartter syndrome caused by mutations in the BSND gene. Histochem Cell Biol 119, 485–493 (2003). https://doi.org/10.1007/s00418-003-0535-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0535-2