Abstract
We summarize in this paper the recently published results on multidrug resistance-associated proteins 3, 4, and 5 (MRPs 3–5). MRP3 can transport organic compounds conjugated to glutathione, sulfate, or glucuronate, such as estradiol–17β-glucuronide, bilirubin–glucuronides, and etoposide–glucuronide, and also bile salts and methotrexate. Studies in knockout mice have shown that Mrp3 contributes to the transport of morphine–3-glucuronide and acetaminophen–glucuronide from the liver into blood. There is no evidence for a major role of MRP3 in bile salt metabolism, at least in mice. The function of MRP3 in other tissues, notably the gut and the adrenal cortex, remains to be defined. MRP4 and MRP5 have attracted attention by their ability to transport cyclic nucleotides and many nucleotide analogs. The initial reports that MRP4 and 5 can transport cGMP with μM affinity have not been confirmed in recent work and the physiological importance of cyclic nucleotide transport by MRP4 and 5 remains to be determined. Transfected cells containing high concentrations of MRP4 and 5 are moderately resistant to base, nucleoside, and nucleotide analogs. The affinity of both transporters for nucleotide analogs is low (K m around 1 mM) and there is no evidence that the transport of these compounds results in resistance in vivo. The physiological function of MRP4 and 5 remains to be found.
Similar content being viewed by others
Introduction
One of the largest sub-families of the ATP-binding cassette (ABC) transporters able to affect drug disposition is the ABCC (multidrug resistance-associated protein, MRP) family. There are now nine members of the multidrug resistance-associated protein family and eight of these (MRP1–8) are known to be organic anion transporters. Between them, the MRPs can transport a large range of organic anions, including anionic drugs and drugs conjugated to glutathione (GSH), sulfate, or glucuronate. No two MRPs have exactly the same substrate specificity or tissue distribution and the precise function of most of the MRPs remains to be established. The MRPs have been reviewed by us [13–19] and others [1, 11, 20, 35, 41, 46, 50, 54, 55, 71, 78] in recent years and a comprehensive review by Deeley et al. is in press in Physiological Reviews (2006). In this paper, we discuss recent data on MRP3, 4, and 5. The other MRPs are discussed in other reviews in this volume.
MRP3 (ABCC3)
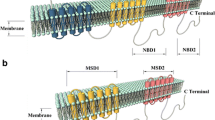
MRP3 consists of 1,527 amino acids [8] and its predicted membrane topology resembles that of MRP1. Among the MRP family members, MRP3 shares the highest degree of amino acid homology with MRP1 (58%) [8, 47, 53]. MRP3 was, therefore, initially expected to transport a similarly broad spectrum of anti-cancer agents as MRP1 and to contribute to clinically relevant drug resistance. The early finding that rat Mrp3 is able to transport some bile salts also led to speculations that MRP3 would be involved in bile salt metabolism. Neither of these conjectures was borne out of experimental data, however. It now appears that MRP3 is involved in transporting some endogenous glucuronosyl conjugates, such as bilirubin–glucuronides, and conjugated drugs out of cells, but other functions may still turn up.
Substrate specificity of MRP3
MRP3 is a typical organic anion transporter able to transport organic compounds conjugated to GSH, sulfate, or glucuronate. The vesicular transport system has been extensively used to characterize its substrate specificity (Table 1). MRP3 has a preference for glucuronidated compounds with morphine–3-glucuronide (M3G) [111], etoposide–glucuronide [110], estradiol–17β-glucuronide (E217βG) [37, 110, 114], and bilirubin–glucuronides [9, 60, 112] being examples of excellent substrates. MRP3/Mrp3 transports several bile salts although the actual rate of transport is species dependent with rat Mrp3 transporting several bile salts with high affinity and at a high rate [38], whereas human MRP3 is less proficient in this respect [109]. However, the catalytic efficiencies (V max/K m) for the transport of the glucuronidated bile salts hyocholate–glucuronide and hyodeoxycholate–glucuronide by human MRP3 are the highest reported for any MRP3 substrate to date [112]. The physiological significance of this high efficiency remains to be tested.
Unlike MRP1 and MRP2, MRP3 appears unable to transport GSH [53]. Cells with high MRP3 levels contain normal GSH concentrations and transport of neutral natural product agents by MRP3 does not seem to require GSH, as buthionine sulfoximine does not attenuate MRP3-mediated resistance to etoposide [110]. The affinity of MRP3 for methotrexate (MTX) is low [37, 110, 114], consistent with the fact that MRP3 only confers resistance during short-term exposure to high MTX concentrations [53].
Transport by MRP1 and MRP2 can be stimulated by several compounds [10, 26, 107] and this also holds for MRP3. Compounds shown to increase MRP3-mediated transport of E217βG are the sulfate conjugates ethinyl estradiol–sulfate (fourfold) [26], E3040 sulfate (1.5-fold) [37], and 4-methylumbelliferone–sulfate (fivefold) [37]. Whether stimulated transport has any physiological relevance [18] remains to be seen, however.
Tissue distribution and induction of MRP3
Compared to MRP1, MRP3 has a restricted tissue distribution pattern. MRP3 is expressed in liver, pancreas, small intestine, and colon with lower amounts of mRNA detected in bladder, kidney, pancreas, lung, spleen, stomach, and tonsils [8, 47, 51, 53]. The presence of MRP3 protein has been confirmed in liver, kidney, the intestine, adrenals, pancreas, gallbladder, pancreas, and spleen [86]. In polarized epithelial cells, MRP3 localizes to the basolateral membrane [86]. There is controversy about the actual amount of MRP3 that is present in human liver. Several studies detected high levels of MRP3 mRNA in human liver [8, 47, 51, 52]. Immunohistochemistry of normal human liver, however, showed only prominent MRP3 staining in cholangiocytes with some weak staining of the hepatocytes around the portal tract [86]. These contrasting results might be explained by a recent study of Lang et al. [57] who found that hepatic MRP3 expression varies (up to 80-fold) among individuals. Part of this variation could be linked to a single nucleotide polymorphism in the promotor region of MRP3 leading to diminished binding of nuclear factors. In normal rat liver, Hirohashi et al. [36] only detected low Mrp3 mRNA levels, whereas in mouse liver, Zelcer et al. [112] found high Mrp3 levels by immunoblot and immunohistochemistry analysis. This difference between rat and mouse may be partly strain dependent, as we recently found that hepatic Mrp3 levels vary among different mouse strains (unpublished results).
MRP3 in the liver is highly inducible. In humans [86, 116] and rats [30, 36, 70], MRP3/Mrp3 is up-regulated during cholestasis and in the absence of functional MRP2/Mrp2, as seen in patients with the Dubin–Johnson syndrome [51] and Eisai hyperbilirubinemic rats [36]. This shows that MRP3 is induced in these species when the canalicular route for the excretion of organic anions is blocked. In mice, the inducibility of Mrp3 is less pronounced, with no or modest up-regulation during bile duct ligation (BDL), depending on the mouse strain [12, 112], or in the absence of Mrp2 (Marijn Vlaming and Alfred Schinkel, personal communication). As basal levels of Mrp3 are high in mice compared to rats, there may be little room for up-regulation.
The induction of MRP3 in the liver is thought to proceed via receptor-mediated pathways including several nuclear receptors [33, 39, 62, 105]. An important nuclear receptor is the pregnane-X-receptor (PXR). Hepatic induction of Mrp3 by PXR activators is absent in Pxr knockout (KO) mice [95], indicating that this nuclear receptor regulates Mrp3 expression. The involvement of the constitutive androstene receptor (CAR) in MRP3 induction is doubtful as Cherrington et al. [25] still found hepatic Mrp3 induction in Car KO mice treated with the prototype CAR activator phenobarbital. The direct involvement of other nuclear receptors in the induction of MRP3 in the liver remains to be demonstrated.
The induction of MRP3 is not restricted to the liver as several bile salts have been shown to induce the expression of MRP3 in an enterocyte cell line (Caco2) as well as in the murine colon [40].
What is the in vivo role of MRP3?
Based on the basolateral localization of MRP3 in liver and intestine [82, 86], several functions have been proposed for this transporter. The liver and intestine play a key role in the enterohepatic circulation of bile acids as well as in the detoxification of endogenous and exogenous compounds through biotransformation and subsequent excretion. MRP3 was hypothesized to play a role in bile salt physiology because rat Mrp3 transports several conjugated bile salts with high affinity and at considerable rate [38] and hepatic MRP3/Mrp3 is up-regulated during cholestasis [36, 86, 116]. The recent generation of Mrp3 KO mice has made it possible to study this hypothesis more directly. Two groups independently found that there was no difference in serum levels of bile salts and liver damage between Mrp3 KO and WT mice after BDL [9, 112], arguing against a prominent role of murine Mrp3 in the basolateral efflux of major bile salts during BDL. This result in mice obviously does not exclude such a role for MRP3/Mrp3 in other species. However, as human MRP3 transports taurocholate with low affinity and at a low rate [109], we expect that MRP3 does not protect the human liver during cholestasis either.
In contrast, glucuronidated hyocholate (HC) and hyodeoxycholate (HDC) are excellent substrates for human MRP3 [112]. These bile salts are formed in humans [75, 83], but not in mice. Liver perfusion experiments with HDC demonstrated that murine Mrp3 significantly contributes to the basolateral excretion of HDC–glucuronide. MRP3 may, therefore, be involved in the excretion of these minor bile salts from the liver.
MRP3 was also thought to play a role in the uptake of bile salts in the ileum and, thereby, to contribute to their enterohepatic circulation. However, Mrp3 KO mice have no abnormalities in taurocholate uptake in the ileum and total fecal bile salt excretion is similar to that of WT control animals [112]. This demonstrates that there is no role for Mrp3 in the enterohepatic circulation of bile salts, at least in mice. Cholestasis results in increased serum levels not only of bile salts but also of (intrahepatically formed) bilirubin–glucuronides. It is interesting that Mrp3 KO mice have a diminished ability to excrete bilirubin–glucuronides from the liver into the circulation during BDL, resulting in lower serum bilirubin–glucuronide levels [9, 112]. MRP3 might, therefore, provide an alternative excretion route from the liver when normal canalicular excretion of bilirubin–glucuronide is blocked. Finding other physiological functions of MRP3 in the disposal of endogenous metabolites clearly needs more work. The Mrp3 KO mice have no overt phenotype and the high concentration of MRP3 in two of the three zones of the adrenal cortex [86] remains unexplained.
Recent papers show, however, that MRP3 has a defense function and contributes to the excretion of toxic organic anions. The preference of MRP3 for glucuronidated compounds combined with its presence in tissues that have a high glucuronidating capacity—like the intestine, kidney and especially the liver—suggested that this transporter has a role in the disposal of xenobiotics and metabolic waste products after their conjugation to glucuronic acid [96]. In agreement with this function is the coordinate induction of MRP3 in the liver along with metabolizing enzymes by several compounds [62, 95]. The transport of acetaminophen– [64], morphine– [111] and hyodeoxycholic acid glucuronide [112] from the liver to the circulation is indeed virtually absent in Mrp3 KO mice. This results in strongly decreased plasma levels and decreased urinary excretion of these glucuronides. The block in M3G excretion only leads to a modest increase in the concentration of this compound in the liver, as it can be rerouted into the bile. The transporter responsible is MRP2, which is present in the canalicular membrane of the hepatocyte. We have shown that MRP2 can also transport M3G and that in mice lacking Mrp2 excretion into bile is blocked (unpublished observations). Similar results have been obtained with acetaminophen, with mouse Mrp3 [64] and rat Mrp2 [105] mediating transport of acetaminophen–glucuronide from the hepatocyte into circulation and bile, respectively.
These results emphasize the crucial role of MRP2 and MRP3 in the disposal of drug–glucuronide conjugates from the liver, as schematically indicated for morphine in Fig. 1, where Mrp2 and Mrp3 compete for the morphine–glucuronide conjugates in the liver. Note that the affinity of MRP3 for M3G and acetaminophen–glucuronide in vesicular transport assays appears to be low (see Table 1). It is possible that the K m values determined in vitro do not reflect the affinity of MRP3 for these substrates in vivo, for instance, because modulatory compounds not present in vitro decrease the K m in vivo. It is also possible, however, that MRP3 can do its job because MRP3 is a high-capacity pump and because the levels of drug–glucuronide conjugates in liver can reach substantial levels [64, 111].
The induction of MRP3 in the liver can limit the enterohepatic circulation of glucuronidated drugs, resulting in shorter drug exposure. This might result in drug–drug interactions as shown for phenobarbital, which alter the disposal of acetaminophen–glucuronide [90].
MRP3 and multidrug resistance of tumor cells
Whether MRP3 contributes to clinical drug resistance is doubtful. In transfected cells, MRP3 confers low levels of resistance to etoposide [53, 110, 113] and teniposide [53, 110] and higher levels of resistance to MTX in short-time, high-concentration exposures [53]. These low levels of MRP3-mediated resistance might partly be due to the low levels of MRP3 in transfected cells and the presence of other endogenous drug transporters with overlapping drug specificity [110]. One study also found low levels of MRP3-mediated vincristine resistance in transfected HEK293 cells [113]. This result was not confirmed, however, in other cell lines in other laboratories [5, 34, 53]. Several studies have also tried to link MRP3 expression in cancer cell lines and patient-derived tumor samples to resistance against anti-cancerr agents. The results in cancer cell lines were either negative [32, 52] or the resistance spectrum found did not fit the known drug resistance spectrum associated with MRP3 [106]. MRP3 has been detected in solid as well as in hematological tumors. In pancreatic carcinoma [49] and acute lymphoblastic leukemia [92], tumor grade and survival, respectively, correlated with MRP3 expression. In contrast, in breast carcinoma samples, MRP3 expression neither correlated with anthracyclin resistance nor with clinical outcome [32]. Based on the low levels of resistance found in MRP3-transfected cells and the narrow spectrum of anti-cancerr agents to which MRP3 mediates resistance, we think that the role of MRP3 in clinically relevant drug resistance will turn out to be limited.
MRP4 (ABCC4)
The gene coding for MRP4 was identified in 1996 and is located on the long arm of chromosome 13 at 13q32 [6, 52, 58, 88]. The MRP4 transcript encodes the smallest of the MRP proteins with a length of 1,325 amino acids with a secondary structure resembling that of MRP5. Both MRP4 and MRP5 lack the additional N-terminal spanning domain that is present in MRP1 [14].
Tissue distribution and expression pattern
The expression of the human MRP4 gene was initially reported to be restricted to a few tissues [52]. However, subsequent work demonstrated that MRP4 is widely expressed with mRNA levels ranging from very high in prostate to barely detectable in liver [58]. In fact, a screening of the human expressed sequence tag database against the MRP4 transcript shows that MRP4 mRNA is present in almost all tissues except bone marrow, cervix, thymus, vascular endothelium, and soft tissue (http://www.ncbi.nlm.nih.gov). Immunostaining localized the MRP4 protein to the kidney [97], liver [80], erythrocytes [48], adrenal gland [108], platelets [43], brain [67], and pancreas [49] in humans. In rats and mice, Mrp4 mRNA and protein was also found in many tissues with highest levels in kidney [21, 61, 63]. Mrp4 protein levels in mouse liver are low, and both liver and kidney Mrp4 levels are higher in females than in males [7, 62, 63, 87]. Whether this gender difference also holds for human liver and kidney remains to be investigated.
A unique feature of MRP4 is its dual localization in polarized cells: it can be present apically as well as basolaterally depending on the cell type. Lee et al. [59] localized MRP4 to the basolateral membrane of the tubulo-acinar cells of the human prostate by immuno-histochemistry. In contrast, MRP4 was found in the apical membrane in the proximal tubule brush border in the kidney [91, 97]. A basolateral location was also observed in human, rat, and mouse hepatocytes [80] and in pancreatic ductular epithelial cells [49]. In human and murine brain, MRP4 is present in the basolateral membrane of the choroid plexus epithelium and also at the luminal side of capillary endothelium [21, 61, 67]. However, in primary cultured bovine brain microvessel endothelial cells, MRP4 was equally distributed over the apical and basolateral plasma membrane [115]. In conclusion, the location of MRP4 in the membrane is cell and tissue specific, allowing this transporter to be involved in the efflux of substrates into the blood and urine as well as towards adjacent cells into the extracellular matrix or stroma. MRP4 has also been detected within the membrane of dense delta-granules in human platelets, albeit with only a single polyclonal antibody [43]. The role of MRP4 in these granules remains to be determined.
MRP4 substrate specificity
Schuetz et al. [88] showed that cells selected for resistance against the nucleotide analog, 9-(2-phosphonylmethoxyethyl)adenine (PMEA), overproduced MRP4 and subsequent work has established that MRP4 can confer cellular resistance to a range of base, nucleoside, and nucleotide analogs [2, 23, 24, 56, 76, 100]. Cytotoxicity assays performed on cell lines transfected with MRP4 cDNA constructs have established a wide range of possible substrates amongst anti-cancer and anti-viral compounds (Table 2). The most recent additions to this list are the plant polyphenols resveratrol and quercetin [103]. In the case of the established MRP4 substrates, 6-thioguanine (6TG) and PMEA, drug resistance is due to transport of the nucleoside monophosphate, but not the base, nucleoside, nucleoside diphosphate, or triphosphate [76, 100]. Nucleoside monophosphates are organic anions and, presumably, this is the drug version that is transported also in the case of the other nucleoside/base analogs in the MRP4 resistance spectrum (Table 2).
Cyclic nucleotide transport by MRP4
Multidrug resistant proteins, MRP4, 5, and 8 transport cyclic nucleotides (reviewed in [84]). Although an initial report suggested that MRP4 is a high-affinity cGMP transporter [24], this was not confirmed by later work (see Table 1). An accumulating body of indirect evidence suggests that MRP4 is the main transporter responsible for the efflux of messenger cGMP from erythrocytes [24, 48, 85, 89, 93, 104]. However, all experiments were conducted in vitro with inside-out membrane vesicles prepared from either cultured cell lines or erythrocytes. cGMP efflux from intact erythrocytes has not yet been demonstrated and there is no evidence that mature erythrocytes can actually make cGMP. The physiological relevance of cGMP efflux by MRP4 from erythrocytes is, therefore, questionable, especially as MRP4 transports cGMP with low affinity [48, 85, 101]. In view of the observed high rate of cGMP transport by erythrocyte vesicles, MRP4 might limit the ability of erythrocytes to accumulate base/nucleoside analogs. Erythrocytes may function as a carrier system in the transport of endogenous compounds and xenobiotics, such as anti-cancer agents 6-mercaptopurine (6MP) and 6TG, through the body. Active low affinity, high capacity, and efflux of these compounds and their metabolites from the erythrocyte by MRP4 might, therefore, limit the bioavailability of these drugs [31].
MRP4 is present at high levels in the apical membrane in human kidney brush border proximal tubules [97] and is, therefore, a candidate for the active secretion of cGMP and cAMP in urine. However, a recent publication by Chen et al. [22] shows a twofold increase of Mrp4 protein in kidneys of Mrp2-deficient TR− rats without the expected increase in excreted cAMP (cGMP was not measured). Moreover, another group reported that the down-regulation of Mrp4 in rat kidney under cholestatic conditions coincides with an increase in secreted cAMP in urine [29]. These two studies do not support an important role for renal Mrp4 in the excretion of urinary nucleotides, even though the authors postulate that Mrp4 is, in fact, the main cAMP transporter. The Mrp4 KO mouse [61] might help to clarify this issue.
What is the function of MRP4?
Vesicular uptake experiments (summarized in Table 1) with inside-out vesicles prepared from cells transfected with MRP4 cDNA constructs have identified two potential physiological MRP4 substrates: bile salts [79, 80] and urate [98]. The transport of the bile salts taurocholate, cholate, and glycocholate was dependent on the presence of GSH (analogs). Such dependence has not been observed for other MRP4 substrates [48, 77, 101, 108]. Given the relatively low rates of bile salt and urate transport observed, it would be important to verify the postulated role of MRP4 in the transport of these substrates in Mrp4 KO mice.
Although hepatic MRP4 protein levels are relatively low, they increase in mice and rats under hepatic stress [3, 4, 29]. This was first seen in Fxr KO mice, which have reduced levels of the major canalicular bile salt pump, BSEP, and increased hepatic Mrp4 mRNA [87]. In all models, the plasma and urine levels of bile salts and/or cAMP were elevated. In contrast, the Mrp4 levels in the livers of Mrp2-deficient rats are not elevated [22, 44]. Acute hepatotoxic stress and hyperbilirubinemia due to Mrp2 deficiency obviously activate different compensatory routes.
A class of endogenous substrates to MRP4 that is structurally related to the bile salts is represented by conjugated steroids such as E217βG [24] and dehydroepiandrosterone-3-sulphate (DHEAS) [108]. A high-affinity transport of prostaglandin E1 (PGE1) and PGE2 [77] and other prostanoids [81] was also demonstrated. Whether the transport of conjugated steroids and prostanoids by MRP4 is physiologically important remains to be determined.
In human and murine brain, MRP4 is mainly present in the capillary endothelium, but MRP4 has also been detected in the basolateral membrane of the choroid plexus epithelium and in astrocytes [21, 61, 67]. These locations of MRP4 indicate that it may contribute to the barrier function at the blood–brain barrier (apically in capillary endothelium) and at the blood–cerebrospinal fluid barrier (basolaterally in choroid plexus epithelium). An initial report which investigates the putative barrier function for MRP4 seems to confirm this hypothesis; levels of the anti-cancerr agent topotecan in brains from Mrp4 KO mice were higher than in those from WT mice [61]. The presence of MRP4 in the astrocytic membrane implies that MRP4 may also be involved in the transport of endogenous substrates in brain (e.g., cGMP, cAMP, and GSH). The role for MRP4 in the efflux of drugs and other compounds from the brain and its importance in the efflux of cyclic nucleotides and GSH from astrocytes and possibly other cells in the brain remain to be investigated.
MRP4 and multidrug resistance of tumor cells
Although MRP4 is present in many human cancer cell lines [52], there is no conclusive evidence linking MRP4 to drug resistance in human tumors. To date, only a single report by Norris et al. [69] suggests a correlation between increased MRP4 levels and tumor prognosis in patients with primary neuroblastoma. The authors attribute this correlation to the ability of MRP4 to transport irinotecan and its active metabolite SN38 (Table 2).
MRP5 (ABCC5)
Initial studies
Early work on MRP5, summarized by [13, 15, 16, 35], showed that MRP5 is a typical organic anion pump transporting acidic organic dyes [65] (but not calcein), DNP–GS, and GSH [102] and inhibited by sulfinpyrazone and benzbromarone [102]. Like MRP4 and MRP8, MRP5 stands out through its ability to transport nucleoside monophosphate analogs [102]. Where tested, neither the corresponding nucleoside nor the nucleoside di- and triphosphates are transported.
An analysis of tissue RNA indicated that MRP5 is ubiquitously expressed, albeit at a low level, as the available antibodies only barely detected the protein in tissues. In transfected epithelial cells, MRP5 routes to the basolateral membrane [102].
Cyclic nucleotide transport by MRP5
Jedlitschky et al. [42] discovered that MRP5 transports the cyclic nucleotides 3′, 5′-cAMP and -cGMP. In transport experiments with vesicles of membranes derived from V79 hamster lung fibroblasts transfected with a human MRP5 cDNA construct, they obtained K m values of 2.1 μM for cGMP and 379 μM for cAMP. Interestingly, cGMP transport was highly sensitive to inhibitors of cGMP phosphodiesterase, such as zaprinast, trequinsin, and sildenafil. Trequinsin was found to inhibit cGMP transport competitively with a K i of 240 nM.
Although other labs have confirmed the ability of MRP5 to transport cGMP, they obtained a much lower affinity for this substrate. cGMP was found to compete with other MRP5 substrates for transport, but half-maximal inhibition required concentrations of about 1 mM, not in micromolars [74, 76]. Low-affinity MRP5-mediated efflux of cyclic nucleotides was also observed from intact HEK cells in which intra-cellular cGMP production was stimulated with nitroprusside or cAMP production with forskolin [101]. The authors conclude “that MRP5 is a low-affinity cyclic nucleotide transporter that may at best function as overflow pump, decreasing steep increases in cGMP levels under conditions where cGMP synthesis is strongly induced and phosphodiesterase activity is limiting” [101].
The high sensitivity of MRP5 to cGMP phosphodiesterase inhibitors, reported by Jedlitschky et al. [42], has not been confirmed in other labs either. In vesicular transport experiments with alaninyl-d4TMP as substrate, Reid et al. [76] saw only marginal inhibition by 1 μM zaprinast and no inhibition by 1 μM sildenafil or trequinsin. Half-maximal inhibition of 5-FdUMP transport by these drugs required 20 μM or more [74].
It is unlikely that the discrepancy between the results of Jedlitschky et al. [42] and of other labs [74, 76, 101] is due to MRP5 polymorphisms. The cDNA construct used in [42] was kindly provided to us by Dr. Jedlitschky. In intact HEK293 cells, this construct produced MRP5 able to extrude cGMP from the cells, but only with low affinity (I. van der Heijden and P. Borst, unpublished). We think that the high-affinity, sildenafil-sensitive cGMP transport observed in V79 hamster cells by Jedlitschky et al. [42] may have been due to up-regulation of an endogenous transporter. As these cells are not available to other investigators, this conjecture [101] cannot be verified.
Transport of nucleotide analogs by MRP5
One of the characteristic properties of MRP5, which it shares with MRP4 and MRP8, is its ability to transport nucleoside monophosphate analogs. Table 2 summarizes the data available for resistance of MRP5-transfected cells against the analogs. Table 1 presents the results of published vesicular transport experiments. A problem in presenting these results is that the two labs in which these experiments were done get different results, notwithstanding the use of MRP5 constructs with identical sequences introduced in the same cell type, HEK293. A reasonable explanation for the discrepancy is that the single transfectant studied in the Eli Lilly lab has more functional MRP5 in the cellular plasma membrane than the 5I clone used for most experiments in the Borst lab. Clones with a high level of MRP5 in the plasma membrane are not easily obtained, as a major unpublished screening effort in the Borst lab has failed to turn up MRP5 transfectants with resistance levels as high as those of clone 36 of the Lilly group [74].
Vesicular transport experiments with MRP5 made a sluggish start because none of the standard MRP substrates worked. Reid et al. [76] (Table 1) then found transport of alaninyl-d4TMP, but this was not a convenient substrate because so little was available that no K m or V max could be determined. Nevertheless, this substrate was useful for competition experiments, which showed that MRP5 has a very low affinity for the nucleotide analogs that it transports (Table 3) [76], confirming earlier conclusions from experiments with intact cells [100]. Several other useful substrates that are readily available were found more recently (Table 1), notably MTX and the dye dicarboxy-dichlorofluorescein diacetate (CDCF) [73]. The only substrate with micromolar affinity is CDCF. The affinity of MRP5 for nucleotide analogs, for MTX and its analogs, and for folate is low.
The only “natural” nucleoside monophosphate that interacts with MRP5 is dUMP (Table 3), although with a very low affinity. The inhibition by 5-FudR (Table 3) is also unusual and the only example of a nucleoside analog interacting directly with MRP5 rather than after conversion into a nucleotide [74, 76, 100, 102]. Note also in Table 1 that MRP5 is able to transport MTX–glucuronide, a talent it shares with BCRP (ABCG2); MRP1–4 transport MTX, but not MTX–glucuronide [99].
Miscellaneous substrates
Long lists of compounds that appear not to be transported by MRP5 have been assembled [65, 74, 76, 102]. McAleer et al. [65] reported weak (about threefold) resistance of MRP5 transfectants against cadmium chloride and antimonyl tartrate (but not arsenite, which usually accompanies antimonite resistance), but this has not been confirmed in a later work. McAleer et al. [65] also found reduced uptake of fluorochromes, such as 5-chloromethylfluorescein diacetate, in MRP5 cells and this has recently been confirmed and extended by Pratt et al. [73], who showed that the closely related CDCF, which is membrane impermeable, is suitable for vesicular transport studies (Table 1).
Pratt et al. [74] find for their MRP5 transfectant a threefold resistance to cisplatin and oxaliplatin and a twofold resistance to doxorubicin, but no cisplation or doxorubicin resistance was observed by McAleer et al. [65] and Wijnholds et al. [102]. Wijnholds et al. [102] conversely found some resistance to etoposide/teniposide not found elsewhere [102]. It is unlikely that MRP5 will obviously contribute to MDR or platin drug resistance in the clinic.
Inhibitors of MRP5
Table 4 summarizes results obtained with intact cells and in vesicular transport experiments. MRP5 is inhibited by the standard organic anion transport inhibitors such as benzbromarone, probenecid, sulfinpyrazone, and MK571, but only at fairly high concentrations. The only compound inhibiting at relatively low concentrations is NPPB, known as a cystic fibrosis transmembrane conductance regulator inhibitor. Unfortunately, none of the compounds in Table 4 qualifies as a specific MRP5 inhibitor.
Some of these compounds were also shown to inhibit cGMP efflux from MRP5–HEK293 cells [101]. The dyes rose Bengal and indocyanine green inhibited >80% at 100 μM and also inhibited MRP4 [101].
The tissue distribution of MRP5
Early studies on MRP5 found MRP5 RNA in all tissues and cell lines tested, but at relatively low levels [52, 102]. Getting more detailed information on the tissue localization of the MRP5 protein has proved to be difficult as an increasingly numerous army of monoclonal antibodies failed to give clear pictures. Even the Mrp5 KO mouse, which provides the ideal negative control, has not been helpful (P. Wielinga, J. Wijnholds, and P. Borst, unpublished).
Additional studies showed the presence of MRP5 RNA in many cell lines [72, 94] and in macrophages [45]. Functional assays have shown export of PMEA from cultured rat microglial cells containing both Mrp4 and 5 [27]. The only MRP5 tissue distribution studies were done by Jedlitschky and co-workers with a single polyclonal antibody directed against the C-terminal 14 amino acids of MRP5 [68]. This antibody detects MRP5 in smooth muscle cells of the human genito-urinary tract and in epithelial cells of ureter and urethra and in blood vessels [68]. In placenta, MRP5 was found in the basal membrane of syncytiotrophoblasts and in and around fetal vessels [66]. In heart, MRP5 was found in cardiomyocytes, endothelial cells, and smooth muscle cell [28]. In the brain, MRP5 was found in astrocytes and in pyramidal neurons. Remarkably, MRP5 was also found at the luminal (apical) side of brain capillary endothelial cells, even though it routes basolaterally in MDCK cells [102]. Whether MRP5 is in other human epithelia is not clear from the papers of Jedlitschky et al. [28, 66–68].
What is the function of MRP5?
The discovery by Jedlitschky et al. [42] that MRP5 transports cGMP with high affinity seemed to provide an appealing function for MRP5, as cells are known to export cGMP under some conditions. This cGMP export function has been elaborated on in later papers by Jedlitschky et al. [28, 66–68] but remains controversial as the high-affinity cGMP transport by MRP5 has not been confirmed in other labs. The possibility that MRP5 contributes to resistance against nucleotide analogs [73, 74, 76, 102] or MTX (analogs) [73, 99] remains open, but, also for these compounds, the affinity of MRP5 is low. The Mrp5 KO mouse and even the Mrp4/5 double KO mouse has no spontaneous phenotype (P. Wielinga, J. Schuetz, J. Wijnholds, and P.Borst, unpublished). For the moment, we have to admit that the function of MRP5 remains unknown and that even plausible conjectures are not available.
Abbreviations
- 6MP:
-
6-Mercaptopurine
- 6TG:
-
6-Thioguanine
- 5-FudR:
-
5-Fluorodeoxyuridine
- ABC:
-
ATP-binding cassette
- BCRP:
-
Breast cancer resistance protein
- BDL:
-
Bile duct ligation
- BSEP:
-
Bile salt export pump
- CAR:
-
Constitutive androstene receptor
- CDCF:
-
Dicarboxy-dichlorofluorescein diacetate
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CMFDA:
-
5-Chloromethylfluorescein diacetate
- DHEAS:
-
Dehydroepiandrosterone-3-sulphate
- DNP–GS:
-
Dinitrophenyl-S-glutathione
- E217βG:
-
Estradiol–17β–glucuronide
- FXR:
-
Farnesoid-X-receptor
- GSH:
-
Glutathione
- HC:
-
Hyocholate
- HDC:
-
Hyodeoxycholate
- HEK293:
-
Human embryonic kidney cells 293
- K i :
-
Inhibitory constant
- K m :
-
Michaelis constant
- KO:
-
Knockout
- MDCK cells:
-
Madin Darby canine kidney cells
- M3G:
-
Morphine–3-glucuronide
- MK571:
-
3-([3-(2-[7-Chloro-2-quinolinyl]ethenyl)phenyl-(3-dimethylamino-3-oxopropyl)-thio-methyl]thio)propanoic acid
- MRP:
-
Multidrug resistance-associated protein
- MTX:
-
methotrexate
- NPPB:
-
5-nitro-2-(3-Phenylpropylamino) benzoic acid
- PGE:
-
Prostaglandin E
- PMEA:
-
9-(2-Phosphonylmethoxyethyl)adenine
- PXR:
-
Pregnane-X-receptor
- SNP:
-
Single nucleotide polymorphism
- V max :
-
Maximal velocity
- WT:
-
Wild type
References
Adachi M, Reid G, Schuetz JD (2002) Therapeutic and biological importance of getting nucleotides out of cells: a case for the ABC transporters, MRP4 and 5. Adv Drug Deliv Rev 54:1333–1342
Adachi M, Sampath J, Lan L-B, Sun D, Hargrove P, Flatley RM, Tatum A, Ziegelmeier MZ, Wezeman M, Matherly LH, Drake RR, Schuetz JD (2002) Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem 277:38998–39004
Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE (2005) Coordinated expression of multidrug resistance associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89(2):370–379
Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE (2005) Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83:44–52
Allen JD, Brinkhuis RF, Van Deemter L, Wijnholds J, Schinkel AH (2000) Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to bassal drug resistance. Cancer Res 60:5761–5766
Allikmets R, Gerrard B, Hutchinson A, Dean M (1996) Characterization of the human ABC superfamily: isolation and mapping of 21 new genes using the expressed sequence tags database. Hum Mol Genet 5:1649–1655
Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279:22250–22257
Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD (1998) Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst 90:1735–1741
Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD (2005) Analysis of the in vivo functions of Mrp3. Mol Pharmacol 68:160–168
Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B (2003) Differential modulation of the human liver conjugate transporters MRP2 and MRP3 by bile acids and organic anions. J Biol Chem 278:23529–23537
Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B (2003) The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett 140–141:133–143
Bohan A, Chen WS, Denson LA, Held MA, Boyer JL (2003) Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3 (Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem 278:36688–36698
Borst P, Balzarini J, Ono N, Reid G, De Vries H, Wielinga P, Wijnholds J, Zelcer N (2004) The potential impact of drug transporters on nucleoside-analog-based antiviral chemotherapy. Antiviral Res 62:1–7
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters, the MRPs. J Natl Cancer Inst 92:1295–1302
Borst P, Oude Elferink R (2002) Mammalian ABC transporters in health and disease. In: Richardson CC, Kornberg R, Raetz CHR, Thorstensen K (eds) Annual review of biochemistry. Science, California, pp 537–592
Borst P, Reid G, Saeki T, Wielinga P, Zelcer N (2003) The multidrug resistance proteins 3–7. In: Holland IB, Kuchler K, Higgins CF, Cole SPC (eds) ABC proteins: from bacteria to man. Elsevier, London, pp 445–458
Borst P, Wielinga P (2005) Pumping out drugs: the potential impact of ABC transporters on resistance to base, nucleoside, and nucleotide analogs. In: Peters GJ (ed) Deoxynucleoside analogs in cancer therapy. Humana, Totowa, NJ, USA
Borst P, Zelcer N, Van de Wetering JK (2006) MRP2 and 3 in health and disease. Cancer Lett (In press)
Borst P, Zelcer N, Van de Wetering K, Poolman B (2006) On the putative co-transport of drugs by multidrug resistance proteins. FEBS Lett 580:1085–1093
Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di Pietro A (2005) Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev 25:453–472
Chen C, Klaassen CD (2004) Rat multidrug resistance protein 4 (Mrp4, Abcc4): molecular cloning, organ distribution, postnatal renal expression, and chemical inducibility. Biochem Biophys Res Commun 317:46–53
Chen C, Slitt AL, Dieter MZ, Tanaka Y, Scheffer GL, Klaassen CD (2005) Up-regulation of Mrp4 expression in kidney of Mrp2-deficient TR− rats. Biochem Pharmacol 70:1088–1095
Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD (2002) Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res 62:3144–3150
Chen Z-S, Lee K, Kruh GD (2001) Transport of cyclic nucleotides and estradiol 17-β-d-glucuronide by multidrug resistance protein 4: resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem 276:33747–33754
Cherrington NJ, Slitt AL, Maher JM, Zhang XX, Zhang J, Huang W, Wan YJ, Moore DD, Klaassen CD (2003) Induction of multidrug resistance protein 3 (mrp3) in vivo is independent of constitutive androstane receptor. Drug Metab Dispos 31:1315–1319
Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R (2004) Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J Pharmacol Exp Ther 309:156–164
Dallas S, Schlichter L, Bendayan R (2004) MRP4- and MRP5-mediated efflux of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) by microglia. J Pharmacol Exp Ther 309:1221–1229
Dazert P, Meissner K, Vogelgesang S, Heydrich B, Eckel L, Bohm M, Warzok R, Kerb R, Brinkmann U, Schaeffeler E, Schwab M, Cascorbi I, Jedlitschky G, Kroemer HK (2003) Expression and localization of the multidrug resistance protein 5 (MRP5/ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am J Pathol 163:1567–1577
Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL (2004) Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol 40:585–591
Donner MG, Keppler D (2001) Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 34:351–359
Dumez H, Reinhart WH, Guetens G, De Bruijn EA (2004) Human red blood cells: rheological aspects, uptake, and release of cytotoxic drugs. Crit Rev Clin Lab Sci 41:159–188
Faneyte IF, Kristel PM, van de Vijver MJ (2004) Multidrug resistance associated genes MRP1, MRP2 and MRP3 in primary and anthracycline exposed breast cancer. Anticancer Res 24:2931–2939
Ghanem CI, Ruiz ML, Villanueva SS, Luquita MG, Catania VA, Jones B, Bengochea LA, Vore M, Mottino AD (2005) Shift from biliary to urinary elimination of acetaminophen glucuronide in acetaminophen pretreated rats. J Pharmacol Exp Ther 315:987–995
Haga S, Hinoshita E, Ikezaki K, Fukui M, Scheffer GL, Uchiumi T, Kuwano M (2001) Involvement of the multidrug resistance protein 3 in drug sensitivity and its expression in human glioma. Jpn J Cancer Res 92:211–219
Haimeur A, Conseil G, Deeley RG, Cole SP (2004) The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab 5:21–53
Hirohashi T, Suzuki H, Ito K, Ogawa K, Kume K, Shimizu T, Sugiyama Y (1998) Hepatic expression of multidrug resistance-associated protein-like proteins maintained in eisai hyperbilirubinemic rats. Mol Pharmacol 53:1068–1075
Hirohashi T, Suzuki H, Sugiyama Y (1999) Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem 274:15181–15185
Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y (2000) ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem 275:2905–2910
Hitzl M, Klein K, Zanger UM, Fritz P, Nussler AK, Neuhaus P, Fromm MF (2003) Influence of omeprazole on multidrug resistance protein 3 expression in human liver. J Pharmacol Exp Ther 304:524–530
Inokuchi A, Hinoshita E, Iwamoto Y, Kohno K, Kuwano M, Uchiumi T (2001) Enhanced expression of the human multidrug resistance protein 3 by bile salt in human enterocytes. A transcriptional control of a plausible bile acid transporter. J Biol Chem 276:46822–46829
Ishikawa T, Tien Kuo M, Furuta K, Suzuki M (2000) The human multidrug resistance-associated protein (MRP) gene family: from biological function to drug molecular design. Clin Chem Lab Med 38:893–897
Jedlitschky G, Burchell B, Keppler D (2000) The multidrug resistance protein 5 (MRP5) functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275:30069–30074
Jedlitschky G, Tirschmann K, Lubenow LE, Nieuwenhuis HK, Akkerman JW, Greinacher A, Kroemer HK (2004) The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 104:3603–3610
Johnson BM, Zhang P, Schuetz JD, Brouwer KL (2005) Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos (In press)
Jorajuria S, Dereuddre-Bosquet N, Naissant-Storck K, Dormont D, Clayette P (2004) Differential expression levels of MRP1, MRP4, and MRP5 in response to human immunodeficiency virus infection in human macrophages. Antimicrob Agents Chemother 48:1889–1891
Keppler D, König J (2000) Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis 20:265–272
Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y (1998) cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3). FEBS Lett 433:149–152
Klokouzas A, Wu CP, Van Veen HW, Barrand MA, Hladky SB (2003) cGMP and glutathione-conjugate transport in human erythrocytes. Eur J Biochem 270:3696–3708
Konig J, Hartel M, Nies AT, Martignoni ME, Guo J, Buchler MW, Friess H, Keppler D (2005) Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer 115:359–367
König J, Nies AT, Cui Y, Keppler D (2003) MRP2, the apical export pump for anionic conjugates. In: Holland IB, Cole SPC, Kuchler K, Higgins CF (eds) ABC proteins: from bacteria to man. Elsevier, pp 423–443
Konig J, Rost D, Cui Y, Keppler D (1999) Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 29:1156–1163
Kool M, De Haas M, Scheffer GL, Scheper RJ, Van Eijk MJT, Juijn JA, Baas F, Borst P (1997) Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologs of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57:3537–3547
Kool M, Van der Linden M, De Haas M, Scheffer GL, De Vree JML, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Oude Elferink RPJ, Baas F, Borst P (1999) MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A 96:6914–6919
Kruh GD, Belinsky MG (2003) The MRP family of drug efflux pumps. Oncogene 22:7537–7552
Kusuhara H, Sugiyama Y (2004) Efflux transport systems for organic anions and cations at the blood–CSF barrier. Adv Drug Deliv Rev 56:1741–1763
Lai L, Tan TMC (2002) Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J 361:497–503
Lang T, Hitzl M, Burk O, Mornhinweg E, Keil A, Kerb R, Klein K, Zanger UM, Eichelbaum M, Fromm MF (2004) Genetic polymorphisms in the multidrug resistance-associated protein 3 (ABCC3, MRP3) gene and relationship to its mRNA and protein expression in human liver. Pharmacogenetics 14:155–164
Lee K, Belinsky MG, Bell DW, Testa JR, Kruh GD (1998) Isolation of MOAT-B, a widely expressed multidrug resistance-associated protein canalicular multispecific organic anion transporter-related transporter. Cancer Res 58:2741–2747
Lee K, Klein-Szanto AJP, Kruh GD (2000) Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst 92:1934–1940
Lee YM, Cui Y, Konig J, Risch A, Jager B, Drings P, Bartsch H, Keppler D, Nies AT (2004) Identification and functional characterization of the natural variant MRP3–Arg1297His of human multidrug resistance protein 3 (MRP3/ABCC3). Pharmacogenetics 14:213–223
Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24:7612–7621
Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD (2005) Induction of the Mrp family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33:956–962
Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD (2005) Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33:947–955
Manautou JE, De Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO (2005) Altered disposition of acetaminophen in mice with a disruption of the multidrug resistance associated protein 3 gene. Hepatology 42:1091–1098
McAleer MA, Breen MA, White NL, Matthews N (1999) pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem 274:23541–23548
Meyer zu Schwabedissen HE, Grube M, Heydrich B, Linnemann K, Fusch C, Kroemer HK, Jedlitschky G (2005) Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol 166:39–48
Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D (2004) Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience 129:349–360
Nies AT, Spring H, Thon WF, Keppler D, Jedlitschky G (2002) Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J Urol 167:2271–2275
Norris MD, Smith J, Tanabe K, Tobin P, Flemming C, Scheffer GL, Wielinga P, Cohn SL, London WB, Marshall GM, Allen JD, Haber M (2005) Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol Cancer Ther 4:547–553
Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y (2000) Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol 278:G438–G446
Pauli-Magnus C, Meier PJ (2003) Pharmacogenetics of hepatocellular transporters. Pharmacogenetics 13:189–198
Pfrunder A, Gutmann H, Beglinger C, Drewe J (2003) Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1–MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol 55:59–66
Pratt S, Chen V, Perry WI3, Starling JJ, Dantzig AH (2005) Kinetic validation of the use of carboxydichlorofluorescein as a drug surrogate for MRP5-mediated transport. Eur J Pharm Sci (In press)
Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W III, Dantzig AH (2005) The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 4:855–863
Radominska-Pyrek A, Zimniak P, Irshaid YM, Lester R, Tephly TR, St Pyrek J (1987) Glucuronidation of 6 alpha-hydroxy bile acids by human liver microsomes. J Clin Invest 80:234–241
Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P (2003) Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 63:1103
Reid G, Wielinga P, Zelcer N, Van der Heijden I, Kuil A, De Haas M, Wijnholds J, Borst P (2003) The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A 100:9244–9249
Renes J, De Vries EGE, Jansen PLM, Müller M (2000) The (patho)physiological functions of the MRP family. Drug Resist Updat 3:289–302
Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D (2005) Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol (In press)
Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D (2003) Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 38:374–384
Rius M, Thon WF, Keppler D, Nies AT (2005) Prostanoid transport by multidrug resistance protein 4 (mrp4/abcc4) localized in tissues of the human urogenital tract. J Urol 174:2409–2414
Rost D, Mahner S, Sugiyama Y, Stremmel W (2002) Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol 282:G720–G726
Sacquet E, Parquet M, Riottot M, Raizman A, Jarrige P, Huguet C, Infante R (1983) Intestinal absorption, excretion, and biotransformation of hyodeoxycholic acid in man. J Lipid Res 24:604–613
Sager G (2004) Cyclic GMP transporters. Neurochem Int 45:865–873
Sager G, Orbo A, Pettersen RH, Kjorstad kE (1996) Export of guanosine 3′,5′-cyclic monophosphate (cGMP) from human erythrocytes characterized by inside-out membrane vesicles. Scand J Clin Lab Invest 56:289–293
Scheffer GL, Kool M, De Haas M, De Vree JML, Pijnenborg ACLM, Bosman DK, Oude Elferink RPJ, Van der Valk P, Borst P, Scheper RJ (2002) Tissue distribution and induction of human MRP3. Lab Invest 82:193–201
Schuetz EG, Strom S, Yasuda K, Lecurier V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ (2001) Disrupted bile acid homeostais reveals an unexpected interaction among nuclear receptors, transporters and cytochrome P450. J Biol Chem 276:39411–39418
Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A (1999) MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med 5:1048–1051
Schultz C, Vaskinn S, Kildalsen H, Sager G (1998) Cyclic AMP stimulates the cyclic GMP-egression pump in human erythrocytes: effects of probenecid, verapamil, progresterone, theophylline, IBMX, forskolin, and cyclic AMP on cyclic GMP uptake and association to inside-out vesicles. Biochemistry 37:1161–1166
Slitt AL, Cherrington NJ, Maher JM, Klaassen CD (2003) Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metab Dispos 31:1176–1186
Smeets PH, Van Aubel RA, Wouterse AC, van den Heuvel JJ, Russel FG (2004) Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol 15:2828–2835
Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, Haefer R, Zintl F, Sauerbrey A (2003) The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood 102:4493–4498
Sundkvist E, Jaeger R, Sager G (2002) Pharmacological characterization of the ATP-dependent low Km guanosine 3′,5′-cyclic monophosphate (cGMP) transporter in human erythrocytes. Biochem Pharmacol 63:945–949
Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, Weinstein JN, Gottesman MM (2004) Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell 6:129–137
Teng S, Jekerle V, Piquette-Miller M (2003) Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos 31:1296–1299
Tukey RH, Strassburg CP (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616
Van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG (2002) The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13:595–603
Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG (2005) Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol 288:F327–F333
Wielinga P, Hooijberg JH, Gunnarsdottir S, Kathmann I, Reid G, Zelcer N, Van der Born K, De Haas M, Van der Heijden I, Kaspers G, Wijnholds J, Jansen G, Peters G, Borst P (2005) The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res 65:4425–4430
Wielinga PR, Reid G, Challa EE, Van der Heijden I, Van Deemter L, De Haas M, Mol C, Kuil AJ, Groeneveld E, Schuetz JD, Brouwer C, De Abreu RA, Wijnholds J, Beijnen JH, Borst P (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol 62:1321–1332
Wielinga PR, Van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P (2003) Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278:17664–17671
Wijnholds J, Mol CAAM, Van Deemter L, De Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P (2000) Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleoside analogs. Proc Natl Acad Sci U S A 97:7476–7481
Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA (2005) Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 272:4725–4740
Wu CP, Woodcock H, Hladky SB, Barrand MA (2005) cGMP (guanosine 3′,5′-cyclic monophosphate) transport across human erythrocyte membranes. Biochem Pharmacol 69:1257–1262
Xiong H, Suzuki H, Sugiyama Y, Meier PJ, Pollack GM, Brouwer KL (2002) Mechanisms of impaired biliary excretion of acetaminophen glucuronide after acute phenobarbital treatment or phenobarbital pretreatment. Drug Metab Dispos 30:962–969
Young LC, Campling BG, Voskoglou-Nomikos T, Cole SPC, Deeley RG, Gerlach JH (1999) Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res 5:673–680
Zelcer N, Huisman MT, Reid G, Wielinga PR, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P (2003) Evidence for two interacting ligand binding sites in human MRP2 (ATP binding cassette C2). J Biol Chem 278:23538–23544
Zelcer N, Reid G, Wielinga P, Kuil A, Van der Heijden I, Schuetz JD, Borst P (2003) Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP)4 (ATP-binding cassette C4). Biochem J 371:361–367
Zelcer N, Saeki T, Bot I, Kuil A, Borst P (2003) Transport of bile acids in multidrug resistance protein 3 over-expressing cells co-transfected with the ileal sodium-dependent bile acid transporter. Biochem J 369:23–30
Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P (2001) Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3). J Biol Chem 276:46400–46407
Zelcer N, Van de Wetering JK, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH, Borst P (2005) Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A 102:7274–7279
Zelcer N, Van de Wetering K, Waart RD, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, Valk MV, Wijnholds J, Oude Elferink R, Borst P (2005) Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol (In press)
Zeng H, Bain LJ, Belinsky MG, Kruh GD (1999) Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer Res 59:5964–5967
Zeng H, Liu G, Rea PA, Kruh D (2000) Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res 60:4779–4784
Zhang Y, Schuetz JD, Elmquist WF, Miller DW (2004) Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther 311:449–455
Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M (2003) Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol 38:717–727
Acknowledgements
The experimental work in the Borst lab was supported in part by grants of the Netherlands Organization for Scientific Research (NWO program 912-02-93, ZonMW 0401) and of the Dutch Cancer Society (NKI 2001-2473 and 2474).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borst, P., de Wolf, C. & van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch - Eur J Physiol 453, 661–673 (2007). https://doi.org/10.1007/s00424-006-0054-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0054-9