Abstract
Whole-heart multi-parametric optical mapping has provided valuable insight into the interplay of electrophysiological parameters, and this technology will continue to thrive as dyes are improved and technical solutions for imaging become simpler and cheaper. Here, we show the advantage of using improved 2nd-generation voltage dyes, provide a simple solution to panoramic multi-parametric mapping, and illustrate the application of flash photolysis of caged compounds for studies in the whole heart. For proof of principle, we used the isolated rat whole-heart model. After characterising the blue and green isosbestic points of di-4-ANBDQBS and di-4-ANBDQPQ, respectively, two voltage and calcium mapping systems are described. With two newly custom-made multi-band optical filters, (1) di-4-ANBDQBS and fluo-4 and (2) di-4-ANBDQPQ and rhod-2 mapping are demonstrated. Furthermore, we demonstrate three-parameter mapping using di-4-ANBDQPQ, rhod-2 and NADH. Using off-the-shelf optics and the di-4-ANBDQPQ and rhod-2 combination, we demonstrate panoramic multi-parametric mapping, affording a 360° spatiotemporal record of activity. Finally, local optical perturbation of calcium dynamics in the whole heart is demonstrated using the caged compound, o-nitrophenyl ethylene glycol tetraacetic acid (NP-EGTA), with an ultraviolet light-emitting diode (LED). Calcium maps (heart loaded with di-4-ANBDQPQ and rhod-2) demonstrate successful NP-EGTA loading and local flash photolysis. All imaging systems were built using only a single camera. In conclusion, using novel 2nd-generation voltage dyes, we developed scalable techniques for multi-parametric optical mapping of the whole heart from one point of view and panoramically. In addition to these parameter imaging approaches, we show that it is possible to use caged compounds and ultraviolet LEDs to locally perturb electrophysiological parameters in the whole heart.
Similar content being viewed by others
Introduction
In cardiac tissue, transmembrane voltage (V m) and intracellular calcium concentration ([Ca2+]i) are arguably two of the most important parameters of interest in basic cardiac electrophysiology research. The action potential (AP) elicits the rise and fall of intracellular calcium (i.e. calcium transient (CaT)), which regulates contraction in a process known as excitation–contraction coupling [5]. Through complex interactions, both V m and [Ca2+]i influence one another such that perturbations in calcium handling, for instance, have been associated with various cardiac pathologies. As such, optical mapping of V m and [Ca2+]i has become the gold standard [10, 32]. Over the past decade or so, several groups have implemented standard two-camera systems (i.e. separable voltage and calcium dye emissions projected onto two camera sensors), which has yielded much insight into V m and [Ca2+]i dynamics and interplay in the isolated whole heart [8, 16, 24, 25, 29, 32].
Recent developments in light-emitting diodes (LEDs) [2], high-speed cameras and optical filter technologies have ushered in a new era of imaging approaches, which is already impacting optical mapping [12]. Traditionally, excitation light sources have been xenon/halogen/mercury lamps combined with optical filters. These light sources are also usually combined with mechanical shutters and cannot be switched on and off at high-speeds in a simple manner. Replacement bulbs are also costly (e.g. hundreds of dollars). Enter the modern LED. Recent advances in manufacturing processes have yielded high-power LEDs that are low-cost (e.g. tens of dollars) and stable, ranging from deep ultraviolet to infrared. More importantly, light output power can be altered at very high speeds (response times in the nanosecond to microsecond range). In terms of high-speed cameras, frame rates have increased to the point where the so called multi-colour imaging technique [38], which uses a single camera and multi-band emission filter to image multiple parameters, can now be applied to multi-parametric optical mapping. In brief, the multi-colour imaging technique used in this study has three features: (1) the multi-band emission filter has pass-bands for each parameter fluorescence signal, (2) each parameter fluorescence signal emitted by the multi-dye-loaded sample is determined by a unique excitation light source and (3) during any camera frame exposure, only one parameter fluorescence signal is collected [38]. High-speed excitation light switching (as can easily be achieved with LEDs), coordinated with the camera frame exposure and combined with an appropriate multi-band emission filter provides a simple and cheap multi-parametric optical mapping system. Additionally, since a single detector is used, multi-detector co-alignment is not necessary. Recently, this multi-colour technique has been applied to ratiometric V m and ratiometric [Ca2+]i cardiac optical mapping [26]. Although not using multi-band emission filters, single-camera detection of ratiometric V m signals [3] and V m and [Ca2+]i signals [35], similarly using high-speed and coordinated LED excitation light switching, has also been recently reported.
With the recent introduction of novel 2nd-generation voltage dyes, di-4-ANBDQBS and di-4-ANBDQPQ, which provide significant improvements in V m signals (i.e. large ΔF/F), dye loading and internalisation stability, it is desirable to develop multi-parametric optical mapping methods for these dyes [30]. In addition, these new red-shifted dyes have ratiometric properties and they do not give rise to acute toxic effects, or changes in cardiac rhythm in Langendorff-perfused hearts [30]. As reported by Matiukas et al. [30], both di-4-ANBDQBS and di-4-ANBDQPQ exhibit excitation-based ratiometric properties. In effect, on opposite sides of the dye’s excitation isosbestic point, the emission intensity change due to V m change are of opposite polarity. Hence, the isosbestic point is the excitation wavelength at which a change in the V m produces neither a decrease nor increase in emission intensity. This means that if the cardiac tissue is additionally loaded with a calcium dye that has strong absorption at the voltage dye’s isosbestic point, the changes in emission intensity from the dual-dye-loaded sample will only correspond to the CaT. Taking advantage of di-4-ANBDQBS and di-4-ANBDQPQ isosbestic points in multi-parametric optical mapping has yet to be reported. Moreover, as this report will show, the isosbestic points of these two dyes lie in the blue- and green-wavelength range, respectively, which allows both blue- and green-wavelength excitable calcium dyes to be explored.
Besides monitoring V m and changes in [Ca2+]i, monitoring the metabolic state of cardiac tissue using fluorescence methods is attractive in local and global ischemia studies. Imaging of endogenous NADH as a measure of ischemia in the intact isolated heart [33], together with V m/[Ca2+]i has shown that heterogeneous ischemic tissue can lead to ectopic beats and re-entry [22, 37]. Since NADH is excited at relatively deep ultraviolet-wavelengths (340 nm peak absorption), it is possible to combine this imaging with visible-wavelength excited V m and [Ca2+]i optical mapping [22, 37]. Measuring V m, [Ca2+]i and NADH together has yet to be described. As this report will show, the multi-colour imaging technique provides a simple single-sensor solution.
Typical optical mapping systems monitor the electrical activity across the epicardium in just one field of view. In arrhythmias, multiple re-entrant waves have been observed where the cores wander over the epicardium [9, 39]. The need to image all around the heart in such cases motivated the development of panoramic optical mapping systems. With motion-tracking and ratiometric imaging, it may be possible to image the beating heart [15]. Cost-effective single-camera systems [6, 27] and multi-camera systems [13, 21, 31] have been developed to image V m around the whole epicardium. However, a multi-parametric solution (e.g. V m and [Ca2+]i) has not yet been described. Using traditional methods, a three observation site panoramic optical mapping system (ideal in terms of spatial resolution) would require six cameras, an impractical solution for many. Here again, the multi-colour imaging technique provides a simple solution.
Although optical mapping allows one to measure electrophysiological parameters at high spatiotemporal resolution, optical methods to focally perturb cardiac electrophysiology are also desirable. Flash photolysis of caged compounds is one such method [1, 11]. This powerful experimental technique uses ultraviolet light to produce rapid changes in the concentration and/or activity of bioactive signalling molecules. A caged compound is synthesised by taking the bioactive molecule of interest and covalently bonding a light-sensitive masking group (referred to as a cage). Absorption of ultraviolet light releases the cage and changes the molecule’s property. For example, o-nitrophenyl ethylene glycol tetraacetic acid (NP-EGTA) is a calcium buffer that can be inactivated by ultraviolet light. In its caged state, NP-EGTA buffers calcium ions, significantly altering intracellular calcium dynamics. In effect, this compound produces the same calcium buffering effects, when loaded inside cardiac cells, as EGTA [23]. Uncaging the compound with flash photolysis abolishes its buffering action. In effect, flash photolysis of NP-EGTA eliminates the compound’s affinity for calcium ions, returning the CaT back to normal. Flash photolysis of such caged calcium buffers has been applied in isolated cardiomyocytes in combination with calcium fluorescence imaging [28, 36]. AP modulation in neuronal cells by local uncaging of NP-EGTA along the axon has also recently been reported [34]. With the development of powerful LEDs in recent years, uncaging light sources based around ultraviolet LEDs have also been implemented and successfully applied in single cells (e.g. neurons and astrocytes) [4, 17]. Despite its use in isolated cardiomyocytes, application of caged compounds to the whole heart has not yet been reported. Local flash photolysis of such compounds provides a means of studying the effects of electrophysiological heterogeneities. In addition, with the appropriate species (i.e. one with a long AP duration), calcium buffers cannot only be used to alter the CaT but also AP dynamics. Combining such optically based modulation methods with multi-parametric optical mapping could represent a powerful experimental tool.
Hence, ratiometric properties of 2nd-generation voltage dyes coupled with LEDs and the multi-colour imaging technique offers simplified methods for (1) V m and [Ca2+]i, (2) V m, [Ca2+]i and NADH and (3) panoramic V m and [Ca2+]i whole-heart optical mapping. Furthermore, caged compounds can be loaded in the whole-heart and ultraviolet LEDs can be used to focally modulate electrophysiological parameters.
Materials and methods
Isolated Langendorff-perfused rat whole heart
Hearts from female Wistar rats were isolated after cervical dislocation in accordance with Schedule 1 of the UK Home Office Animals (Scientific Procedures) Act of 1986, and swiftly connected to a Langendorff setup (250–350 g; n = 3 di-4-ANBDQBS and fluo-4 optical mapping; n = 3 di-4-ANBDQPQ and rhod-2 optical mapping; n = 2 di-4-ANBDQPQ and rhod-2 and NADH optical mapping; n = 2 di-4-ANBDQPQ and rhod-2 panoramic optical mapping; n = 3 NP-EGTA local uncaging combined with di-4-ANBDQPQ and rhod-2 optical mapping). Hearts were perfused at a constant rate of 5 mL/min with Tyrode’s solution (containing, in mmol/L: NaCl, 140; CaCl2, 1.8; KCl, 5.4; MgCl2, 1; glucose, 11; HEPES, 5; bubbled with oxygen; pH 7.4). All chemicals were obtained from Sigma-Aldrich (Dorset, UK), unless otherwise stated.
Voltage and calcium dye loading
Fluorescent dyes were injected into the aortic cannula for coronary perfusion. For [Ca2+]i imaging, hearts were loaded with dye by re-circulating perfusion with 100 mL of 2.5 μmol/L fluo-4 AM (Life Technologies, Paisley, UK) or rhod-2 AM (Life Technologies) for 30 min. For studies that call for longer [Ca2+]i imaging periods (i.e. over hours), probenecid (Life Technologies) can be added to the perfusate (e.g. 0.2 mM) to reduce extrusion of dye. For V m imaging, hearts were loaded with dye by delivering, without recirculation, a 20-μL bolus of either 29 mmol/L (in pure ethanol) di-4-ANBDQBS or a 20-μL bolus of 27 mmol/L (in pure ethanol) di-4-ANBDQPQ (Richard D. Berlin Center for Cell Analysis and Modeling, University of Connecticut Health Center, USA), applied over 5 min (i.e. diluted in 25 mL perfusate). To load di-4-ANBDQPQ, Pluronic F-127 (Life Technologies) was added to the bolus, to a final concentration of 0.2–0.5 % [30].
Excitation-contraction coupling was blocked for imaging purposes with blebbistatin (Sigma-Aldrich), using a concentration of 10 μmol/L [14]. All experiments were conducted at 36 ± 1 °C.
Multi-parametric optical mapping instrumentation
V m and [Ca2+]i optical mapping (di-4-ANBDQBS and fluo-4 and di-4-ANBDQPQ and rhod-2)
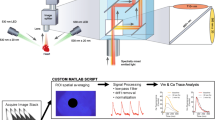
The system implemented here is a modification of the optical mapping approach described before [26], where detailed circuit diagrams, firmware and software code are provided. The primary differences lie in the dye combinations used, and the associated custom-fabricated multi-band emission filters. Below is a brief description of the system (Figs. 1 and 2).
Isosbestic points of di-4-ANBDQBS and di-4-ANBDQPQ. a Di-4-ANBDQBS fluorescence in a rat heart (left and right ventricle view; sinus rhythm), excited with red (red LED filtered with F1) and blue (blue LED filtered with F2a) wavelengths. Fluorescence signals (taken from the 4 × 4-pixel white-square region shown) were collected through the custom-made multi-band filter F3a shown in (c). Illumination with the blue source (Ex2a) yielded no signal during the AP. b Di-4-ANBDQPQ fluorescence in a rat heart (mostly left ventricle view; sinus rhythm), excited with red (red LED filtered with F1) and green (green LED filtered with F2b) wavelengths. Fluorescence signals (taken from the 4 × 4-pixel white-square region shown) were collected through the custom-made multi-band filter F3b shown in (d). Illumination with the green source (Ex2b) yielded no signal during the AP. c Transmission spectrum of a custom multi-band emission filter that passes V m (Em1; di-4-ANBDQBS dye) and [Ca2+]i (Em2a; fluo-4 dye) emitted fluorescence signals. F1 and F2a (for di-4-ANBDQBS and fluo-4 excitation, respectively) excitation filter spectra are shown as dashed curves. d Transmission spectrum of a custom multi-band emission filter that passes V m (Em1; di-4-ANBDQPQ dye) and [Ca2+]i (Em2b; rhod-2 dye) emitted fluorescence signals. F1 and F2b (for di-4-ANBDQPQ and rhod-2 excitation, respectively) excitation filter spectra are shown as dashed curves. Scale bar = 5 mm
V m and [Ca2+]i optical mapping approach using isosbestic points. a System schematic showing key components (see text for details). Since a single camera (combined with a multi-band filter F3a/b) is used, the V m and [Ca2+]i optical mapping system requires no challenging optical alignment. b The multi-colour imaging technique: During any camera frame exposure, either the V m or the [Ca2+]i (Em1 or Em2a/b) signal is acquired by illuminating the dual-dye-loaded tissue with excitation source Ex1 or Ex2a/b, respectively. At sufficiently high camera frame rates and with interpolation, this technique provides a straightforward method of multi-parametric optical mapping. c V m (red) and [Ca2+]i (blue) fluorescence signals (camera signals on a 16-bit scale) taken from the 4 × 4-pixel white-square region shown from a rat heart dual-loaded with di-4-ANBDQBS and fluo-4. d V m (red) and [Ca2+]i (green) fluorescence signals (camera signals on a 16-bit scale) taken from the 4 × 4-pixel white-square region shown from a rat heart dual-loaded with di-4-ANBDQPQ and rhod-2. Scale bar = 5 mm
Excitation of (a) di-4-ANBDQBS/di-4-ANBDQPQ, (b) fluo-4 and (c) rhod-2 are done using:
-
For (a)
LED1, CBT-90-R (peak power output, 32 W; peak-wavelength, 628 nm; Luminus Devices, Billerica, MA, USA); Lens L1, plano-convex lens (LA1951; Thorlabs, Ely, UK); and Filter F1, D640/20X (Chroma Technology, Bellows Falls, VT, USA)
-
For (b)
LED2a, CBT-90-B (peak power output, 53 W; peak-wavelength, 460 nm; Luminus Devices); L2, plano-convex lens (LA1951; Thorlabs); F2a, D440/20X (Chroma Technology) and
-
For (c)
LED2b, CBT-90-G (peak power output, 58 W; peak-wavelength, 524 nm; Luminus Devices); L2, plano-convex lens (LA1951; Thorlabs); F2b, D540/25X (Chroma Technology).
Fluorescence emission from the whole heart is passed through a custom-made multi-band emission filter F3a, ET525/50-800/200M (di-4-ANBDQBS and fluo-4) or F3b, ET585/50-800/200M (di-4-ANBDQPQ and rhod-2), both fabricated by Chroma Technology (now available off the shelf). The filtered fluorescence emission is collected with a ‘fast’ camera lens (f/# 0.95; DO-2595; Navitar, Rochester, NY, USA). Fluorescence images are taken with a high-speed electron-multiplying charge-coupled device camera (EMCCD camera; Cascade 128+; Photometrics, Tucson, AZ, USA). The camera was operated at full resolution (128 × 128 pixels; 510 frames/s). Acquisition software QCapture Pro 6.0 (QImaging, Surrey, BC, Canada), which came with the camera system, was used to configure the camera and to acquire images.
A microcontroller-based interface synchronises excitation light switching with EMCCD camera frame exposures. LEDs are controlled with a custom-built high-power LED driver circuit, as published before [26]. An eight-processor microcontroller (Propeller chip; Parallax, Rocklin, CA, USA) is used to control and coordinate all major components of the set-up (firmware for time-critical tasks was written in assembly language). Communication with a standard desktop computer is achieved with a USB interface module (UM245R; Future Technology Devices International, Glasgow, UK). Custom software written in MATLAB (MathWorks, Natick, MA, USA) was used to communicate with the microcontroller and to perform optical mapping image processing. All electronic components can be acquired from major electronic components distributors (e.g. Digi-Key Corp., Thief River Falls, MN, USA).
V m, [Ca2+]i and ‘ischemia’ optical mapping (di-4-ANBDQPQ and rhod-2 and NADH)
The three-parameter optical mapping approach used the system described above, except that the addition of NADH imaging required a third excitation source (and LED driver) and a different multi-band emission filter (Fig. 3).
V m, [Ca2+]i and ‘ischemia’ optical mapping. a Transmission spectrum of an off-the-shelf triple-band emission filter that passes V m (di-4-ANBDQPQ), [Ca2+]i (rhod-2) and NADH (endogenous) fluorescence, due to excitation with sources Ex1, Ex2 and Ex3, respectively (see text for details). Ex1 and Ex2 excitation filter spectra are shown as dashed curves. b Oscilloscope traces of camera frame-exposure (top trace; 5 V = exposure on) and photodiode signals (bottom trace) recorded from LED excitation sources Ex1, Ex2 and Ex3 (triggered by the frame-exposure signal via a microcontroller). These traces show the non-overlapping on/off cycles of the three excitation sources, and the output stability of the LEDs. c Normalised fluorescence intensity maps (colourbar shown) at progressive time points during sinus rhythm (left and right ventricle view). The delay of the CaT relative to the AP peak (~15 ms delay) is clearly visible. d The change in the normalised fluorescence intensity maps (colourbar shown) of NADH during the course of 15 min before and after left anterior descending artery proximal occlusion (tie-off point marked by a red circle in the bottom panel). Scale bar = 5 mm
Here, excitation of (a) di-4-ANBDQPQ (Ex1), (b) rhod-2 (Ex2) and (c) NADH (Ex3) are done using:
-
For (a)
LED1, CBT-90-R (peak power output, 32 W; peak-wavelength, 628 nm; Luminus Devices); L1, plano-convex lens (LA1951; Thorlabs); F1: D640/20X (Chroma Technology),
-
For (b)
LED2b, CBT-90-G (peak power output, 58 W; peak wavelength, 524 nm; Luminus Devices); L2, plano-convex lens (LA1951; Thorlabs); F2b, S538/19X (Chroma Technology) and
-
For (c)
LED NC4U133 (peak power output, 950 mW; peak wavelength, 365 nm; Nichia, Tokushima, Japan), also collimated with a plano-convex lens (LA1951; Thorlabs).
Fluorescence emission from the whole heart is passed through an off-the-shelf multi-band emission filter, 62007M (Chroma Technology) and collected with the same camera lens as above (f/# 0.95; DO-2595; Navitar).
V m and [Ca2+]i panoramic optical mapping (di-4-ANBDQPQ and rhod-2)
The single-camera panoramic optical mapping system implemented here uses the same general approach as described elsewhere (Fig. 4) [6, 27]. The primary view, S1, is captured directly and the secondary views, S2 and S3, are captured via two back mirrors (ME2-P01; Thorlabs). Because of the large depth-of-focus achieved with a macroscopic camera lens, all three views are simultaneously in focus (in view of the longer light path, mirrored images are of course smaller). Excitation sources for di-4-ANDBQPQ and rhod-2 are the same as described above in ‘ V m and [Ca2+]i optical mapping (di-4-ANBDQBS and fluo-4 and di-4-ANBDQPQ and rhod-2)’. Illumination of all sides of the heart is achieved either directly or via reflection from the back mirrors.
V m and [Ca2+]i panoramic optical mapping using a single camera. a System schematic showing key components (see text for details). A heart-sized model made from Blu-Tak (top right and bottom right) was used to guide positioning of the two back mirrors, lenses and camera. Transmission spectra for filters F1, F2b and F3b are shown in Fig. 1d. Sides S1, S2 and S3 represent projections from three angles, 120° apart, where S1 represents the primary view and S2 and S3 the secondary mirror image views. b V m (red) and [Ca2+]i (green) fluorescence signals (camera signals on a 16-bit scale) taken from the 4 × 4-pixel white-square region shown from a rat heart, in sinus rhythm, dual-loaded with di-4-ANBDQPQ and rhod-2. Top, a grayscale fluorescence image (V m) of sides S1, S2 and S3 (S1 is mostly a view of the left ventricle). The red circle indicates the location of electrical point stimulation. c Normalised fluorescence intensity maps (colourbar shown) at progressive time points during 5 Hz local electrical pacing. The delay of the CaT relative to the AP peak (~21 ms delay) is clearly visible. Scale bar = 5 mm (for primary view S1)
The three projections from the heart (S1, S2 and S3) are imaged through a ‘fast’ camera lens with a short, 17-mm, effective-focal-length (f/# 0.95; DO-1795; Navitar), multi-band filter ET585/50-800/200M (Chroma Technology) and projected to the camera sensor with an image-shrinking (2.5 factor) achromatic relay lens (NT46-005; Edmund Optics, York, UK) to accommodate all images on the camera sensor.
Although whole hearts were imaged in sinus rhythm, we also applied local electrical stimulation to the heart. This illustrated the advantages of a panoramic system in spotting the site of initiation of events not visible from a single point of view. The heart was stimulated at 5 Hz with bipolar pulses (amplitude of 5-15 V, set to 50 % above threshold, for 3 ms duration). A bipolar stimulation electrode (Lohmann Research Equipment, Castrop-Rauxel, Germany) was used, connected to a custom-built stimulator.
Caged compound (NP-EGTA) loading and local flash photolysis (uncaging)
The compound NP-EGTA (AM; Life Technologies) was injected into the aortic cannula for coronary perfusion. Hearts were loaded by re-circulating perfusion with 100 mL at 10 μmol/L, for 1 h. Pluronic F-127 (Life Technologies) was added to a final concentration of 0.2 %. Fluorescent dyes rhod-2 AM and di-4-ANBDQPQ were then loaded (in this order), as described above.
Successful loading of NP-EGTA in the whole heart was evident from calcium optical maps [23]. The mapping system used to image di-4-ANBDQPQ and rhod-2 was the same as described in ‘ V m and [Ca2+]i optical mapping (di-4-ANBDQBS and fluo-4 and di-4-ANBDQPQ and rhod-2)’.
Local uncaging was achieved by focusing, using two plano-convex lenses (LA1951; Thorlabs), the light output from a 365-nm-centred ultraviolet LED (NC4U133; Nichia). Significant uncaging was achieved by locally illuminating the epicardium for 30 s at 10 kHz (50 % duty cycle). The light was pulsed to allow driving of the LED above its maximum rated current (not necessary in these experiments; the on-current was ~1 A, lower than the maximum rating).
Image processing
Custom software written in MATLAB (MathWorks) was used to perform optical mapping image processing. Although time-course signals were left unfiltered (to demonstrate ‘raw’ signal quality), images for normalised fluorescence intensity maps and movies were sometimes filtered using 2D median filtering (MATLAB’s built-in medfilt2 function) to improve image quality.
Results
V m and [Ca2+]i optical mapping (di-4-ANBDQBS and fluo-4; di-4-ANBDQPQ and rhod-2)
For proof-of-principle, we used the isolated rat whole-heart model to validate all methods described in this report. Despite the drawbacks of being small in size and having a short AP duration (e.g. limiting the ability to see effects of NP-EGTA on the AP), the model permitted investigation of multiple technical ‘tricks’. First, we characterised the isosbestic point of di-4-ANBDQBS and di-4-ANBDQPQ, using previously reported studies as a guide [30]. Figure 1a and b show sample results from hearts loaded with di-4-ANBDQBS and di-4-ANBDQPQ, respectively. Excitation at red-wavelengths (longer wavelength than the isosbestic point; red LED filtered with F1) yielded downward-swinging AP signals (top panels), collected through the 700-nm long-pass component of the multi-band filters shown in Fig. 1c, d (black transmission curves; marked Em1). As shown in the bottom panels of Fig. 1a, b, excitation of di-4-ANBDQBS with a blue source (blue LED filtered with F2a; Fig. 1c) and di-4-ANBDQPQ with a green source (green LED filtered with F2b; Fig. 1d) yielded no fluorescence swing due to the AP. These two excitation wavelengths are the excitation isosbestic points for the voltage responses of di-4-ANBDQBS and di-4-ANBDQPQ, respectively.
A schematic of the AP and CaT optical mapping system, taking advantage of the isosbestic points, is presented in Fig. 2a. The system is built from off-the-shelf electronic components, high-power LEDs, optical filters, collimating (L1 and L2) and camera (L3) lenses, and a single high-performance EMCCD camera. The system is similar to that described elsewhere [26], except for the fabrication and use of two high-transmission custom multi-band emission filters F3a and F3b (black transmission curves shown in Fig. 1c, d). Because di-4-ANBDQBS has a blue-wavelength isosbestic point, which lies in the absorption spectra of fluorescein dyes, we combined di-4-ANBDQBS with calcium-dye fluo-4. Multi-band filter F3a (Fig. 1c) was designed to have a pass-band (marked Em2a) significantly overlapping the emission spectra of fluo-4. Similarly, since di-4-ANBDQPQ has a green-wavelength isosbestic point, which lies in the absorption spectra of rhodamine dyes, we combined di-4-ANBDQPQ with calcium-dye rhod-2. Multi-band filter F3b (Fig. 1d) was designed to have a pass-band (marked Em2b) significantly overlapping the emission spectra of rhod-2.
Figure 2b demonstrates the multi-colour principle [38] behind our approach: during one camera frame exposure, V m excitation light (Ex1) is turned on, yielding V m signal collected through the Em1 band (in F3a/b); during the next frame exposure, [Ca2+]i excitation light (Ex2a/b for fluo-4/rhod-2) is turned on, yielding [Ca2+]i signal collected through the Em2a/b band (in F3a/b), etc. Since the calcium dye is excited by the corresponding voltage dye’s isosbestic point, there is no cross-talk between [Ca2+]i and V m signals during any frame exposure. Using a sufficiently fast camera (510 frames/s for this study), with interpolation, one can record V m and [Ca2+]i dynamics simultaneously. Figure 2c and d show results obtained with the di-4-ANBDQBS and fluo-4 and di-4-ANBDQPQ and rhod-2 dye combinations, respectively. Sample V m and [Ca2+]i fluorescence signals from a point on the ventricles are shown for both dye combinations. The signals are unfiltered to demonstrate the quality afforded by the presented system. Longer-term continuous recordings of V m and [Ca2+]i are also possible with this system (see Supplementary Fig. 1 showing multiple 30-s recordings of di-4-ANBDQPQ and rhod-2 activity, obtained over 400 s).
V m, [Ca2+]i and ‘ischemia’ optical mapping (di-4-ANBDQPQ, rhod-2 and NADH)
Extending the multi-colour principle from V m and [Ca2+]i (two parameters) optical mapping, we now describe the implementation of a three-parameter optical mapping system. For proof of principle and motivated by recent work combining NADH imaging with V m/[Ca2+]i optical mapping [22, 37], we implemented a single-camera mapping system to image V m, [Ca2+]i and NADH, simultaneously. Although NADH fluorescence does not undergo rapid changes like V m and [Ca2+]i signals, it has been shown as an indicator for ischemia [33]. Importantly NADH is excited in the ultraviolet-wavelengths and prior optical mapping work has established negligible cross-talk when combined with visible-wavelength voltage and calcium dyes. As described in the methods section above, we used a high-power 365-nm-centred ultraviolet LED (filtering was not required because of the narrow output spectrum) to excite endogenous NADH. The system used is essentially the same as that shown in Fig. 2a, except for (1) the addition of a third excitation source (for NADH) and (2) the use of a triple-band emission filter (F3 in Fig. 3a). Although not having the ideal pass-bands, this filter was off-the-shelf and readily available for these proof-of-principle experiments (i.e. no custom design and fabrication necessary). Here, di-4-ANBDQPQ and rhod-2 dyes were used for V m and [Ca2+]i optical mapping, respectively. As can be seen from Fig. 3a, the last two transmission bands (marked [Ca2+]i and V m) correspond closely to the transmission bands shown in Fig. 1d.
To highlight the versatility afforded by LEDs over traditional light sources (typically combined with mechanical switching), we excited the heart using an excitation sequence representative of the relative dynamics of the V m, [Ca2+]i and NADH (from fast to slow) signals. As can be seen in Fig. 3b, for every seven camera frames, four frames are dedicated to the V m signal, two to the [Ca2+]i signal and only one to the NADH signal. This is just one possible excitation sequence. To change the sequence, the microcontroller firmware simply needs to be changed. Figure 3b shows this excitation sequence in coordination with the camera frame-exposure signal. A simple light detector module, using a high-speed P-type, Intrinsic, N-type photodiode (S5971, Hamamatsu Photonics, Shizuoka, Japan) was built to detect light output from the three excitation sources (Ex1, Ex2 and Ex3 for di-4-ANBDQPQ, rhod-2 and NADH, respectively). The distances between the excitation sources and the photodiode were set so that each source could be distinguished based on the amplitude of the photodiode signal. In addition, LED output powers were set to values typically used in experiments. Figure 3b shows the camera frame-exposure signal (the master signal used by the microcontroller to control LED switching) and photodiode signal acquired on a digital oscilloscope (PicoScope; Pico Technology, St. Neots, UK). The photodiode signal not only demonstrates the non-overlapping turning on and off of the three excitation sources but also shows the output stability of the LEDs.
Figure 3c shows a time series of normalised V m and [Ca2+]i fluorescence intensity maps during sinus rhythm, highlighting the well-known delay (~15 ms in this heart) of the CaT to AP peak (see Supplementary Movie 1). Figure 3d shows the change in the normalised fluorescence intensity maps of NADH over 15 min before and after left anterior descending artery proximal occlusion. As can be seen, regional changes (mostly increases) in NADH fluorescence were seen, which had been observed in previous NADH imaging studies in the rat heart [22, 37]. Heterogeneity of the NADH fluorescence is caused by the slow dynamics of NADH, as well as by regional differences in oxygen supply via collateral circulation [7, 18].
V m and [Ca2+]i panoramic optical mapping (di-4-ANBDQPQ and rhod-2)
Figure 4 shows the design and implementation of a single-camera V m and [Ca2+]i panoramic optical mapping system. Figure 4a shows the instrumentation layout using the same approach described elsewhere [6, 27]. The key advance lies in the utilisation of the multi-colour technique outlined in Fig. 2. Here, the di-4-ANBDQPQ and rhod-2 dye combination was used for panoramic imaging during sinus rhythm and local electrical excitation. As shown in Fig. 4a, a molded model of the heart (Blu-Tak; Bostik, Paris-La Défense, France) was used to guide positioning of the two back mirrors, lenses and camera. A relay lens was used to shrink the image generated by camera lens L3 to fit the camera sensor size. Using a black marker, the heart model was divided into three equal sections, S1, S2 and S3 (numbers 1, 2 and 3 clearly visible in the bottom-right panel). This provided three views, 120° apart. As can be seen in the bottom-right panel, the secondary views, S2 and S3, show images of the heart smaller than the primary view, S1, because these mirror images are at a further distance (compared with S1) from the camera lens. And as can be seen by the orientation of the numbers 2 and 3 on the model (refer to the top-right and bottom-right panels), images S2 and S3 are flipped horizontally. The two back mirrors not only provide secondary views S2 and S3, but also act as reflecting surfaces for illumination. As described elsewhere, a 3D surface profile of the heart can be generated with additional imaging instrumentation (not implemented in the present system) [6, 13, 21, 31].
Figure 4b shows sample V m and [Ca2+]i fluorescence signals from a point on the ventricles of the heart, in sinus rhythm. The point was taken from secondary view S3 and the signals left unfiltered to demonstrate the quality afforded by this single-camera system. Figure 4c shows a time series of normalised V m and [Ca2+]i fluorescence intensity maps during local electrical stimulation, highlighting again the well-known delay (~21 ms in this paced heart) of the CaT to AP peak (see Supplementary Movie 2). Point electrical stimulation was done to underscore the need for panoramic optical mapping systems for spotting events not visible from a single point-of-view.
Caged-compound (NP-EGTA) loading and local flash photolysis (i.e. uncaging)
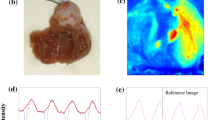
Using a focused 365-nm-centred ultraviolet LED for uncaging in conjunction with the V m and [Ca2+]i (di-4-ANBDQPQ and rhod-2; only rhod-2 data shown) optical mapping system outlined in Fig. 2, we explored the possibility of loading and locally uncaging a caged compound in the whole heart. In this proof-of-principle study, we used NP-EGTA because of its marked effects on the CaT. NP-EGTA, before flash photolysis, acts as a slow calcium ion buffer, decreasing the gain of the calcium-induced calcium release [23]. The left panel of Fig. 5b shows successful loading of this calcium buffer. The CaT (all unfiltered) from three points (left panel, Fig. 5a) on the ventricles clearly shows dramatically modified kinetics (compared, for example, to CaT in Fig. 2b, d). Normalised fluorescence intensity maps of [Ca2+]i are shown at three progressive time points in Fig. 5a (see Supplementary Movie 3 for V m and [Ca2+]i maps).
Loading and local flash photolysis of NP-EGTA in the whole heart. a Left, a grayscale fluorescence image (V m) of the rat heart (mostly right ventricle view). The remaining panels are normalised [Ca2+]i fluorescence intensity maps (colourbar shown) at progressive time points during sinus rhythm, after NP-EGTA loading. b [Ca2+]i (blue, green and red) fluorescence signals (camera signals on a 16-bit scale) taken from 8 × 8-pixel regions (left panel in (a)) from the heart, in sinus rhythm. Left, shows CaT after loading of NP-EGTA. Middle, altered CaT at site 1 (and unaltered CaT at sites 2 and 3) after region 1 local flash photolysis. Right, altered CaT at sites 1 and 2 (and unaltered CaT at site 3) after region 2 local flash photolysis. c Normalised [Ca2+]i fluorescence intensity maps (colourbar shown) at progressive corresponding (to a) time points, highlighting CaT widening at site 1 (left, two panels) and sites 1 and 2 (right, two panels), during sinus rhythm. Scale bar = 5 mm
Local flash photolysis was first performed at site 1. The middle panel of Fig. 5b shows the CaT kinetics changing back to ‘normal’ at site 1 due to inactivation of NP-EGTA’s [Ca2+]i buffering capacity. The left panel of Fig. 5c shows corresponding (to Fig. 5a) normalised fluorescence intensity maps of [Ca2+]i, highlighting CaT widening at the uncaging site (see Supplementary Movie 4). Local flash photolysis was then performed at site 2. The right panel of Fig. 5b shows the CaT kinetics changing back to ‘normal’ at site 2 due to inactivation of NP-EGTA. The right panel of Fig. 5c shows corresponding normalised fluorescence intensity maps of [Ca2+]i, highlighting CaT widening, now at both sites (see Supplementary Movie 5). Control studies using di-4-ANBDQPQ and rhod-2 loaded hearts (but without NP-EGTA) were also done (data not shown) to confirm that the ultraviolet illumination protocol does not visibly damage the tissue (i.e. no changes in V m and [Ca2+]i signals were observed).
Discussion
Here, we presented (1) two V m and [Ca2+]i optical mapping systems using isosbestic points of two 2nd-generation voltage dyes, (2) three-parameter optical mapping (V m, [Ca2+]i and NADH), (3) V m and [Ca2+]i panoramic optical mapping and (4) loading and local flash photolysis of NP-EGTA in the Langendorff-perfused rat whole heart, using a single-camera, LEDs and off-the-shelf components. As such, these methods are scalable and financially viable.
The excitation isosbestic point for di-4-ANBDQBS and di-4-ANBDQPQ is not constant across all species (e.g. guinea pig and rabbit; data not shown). We found the isosbestic point to be at longer wavelength for both the guinea pig and rabbit isolated whole heart. The V m and [Ca2+]i dye combinations presented here still work out, with modifications to calcium-dye excitation wavelength. This variation in the excitation isosbestic point is due to the sensitivity of the dyes’ spectral properties on the molecular composition of the membrane (e.g. cholesterol and saturated lipids have been shown to shift di-4-ANEPPS emission by as much as 60 nm) [19, 20].
The single-camera approach has certain limitations. First, the multi-colour imaging technique depends on the ability to relate observed fluorescence emission changes to a single parameter, when multiple dyes are loaded. This is an inherent problem to all multi-dye investigations. Second, camera frame rates must be high enough to multiplex frame exposures to capture all parameters involved. Based on continuing improvements in the sensitivity and spatiotemporal resolution of cameras, the impact of this limitation will continue to decrease. In addition, as long as sampling rates exceed the physiological parameter dynamics (i.e. fast changes would not be missed at the rate of observation), interpolated signals will give a suitable representation of true signals. Third, in the case of our single-camera panoramic optical mapping system, spatial resolution is reduced compared with a single-sensor approach. In spite of these limitations, such simplified panoramic system permits cardiac electrophysiology studies that are presently impractical from an engineering and financial standpoint.
The use of multiple excitation wavelengths (for different fluorescent probes) during macroscopic imaging of thick tissue preparations, such as the Langendorff-perfused whole heart, makes the interpretation of fluorescence signals difficult. This is because wavelength-dependent differences in tissue penetration depths will cause collection of data from overlapping but non-identical tissue volumes. Although this is minimised in preparations, such as thin ventricular tissue-slices or cardiomyocyte monolayers (for which the present techniques are also applicable), this practical limitation of epifluorescence imaging of thick tissue is a currently unresolved issue. Attempts to put this to scientific use have been reported [15], but we are still far from satisfactory solutions to this issue.
Emitted fluorescence intensity can be highly heterogeneous over the surface of the heart. Regionally differing dye loading and uneven illumination are primary causes for this. The latter can be addressed by use of multiple light sources, to improve uniformity of illumination over the highly curved surface of the heart. As the here presented methods are non-ratiometric, one cannot draw conclusions about absolute V m and [Ca2+]i levels. Still, comparisons of ∆F/F values from the same tissue region before and after an intervention are highly valuable, though, for example in assessing pharmaceutical compound actions.
References
Adams SR, Tsien RY (1993) Controlling cell chemistry with caged compounds. Annu Rev Physiol 55:755–784
Albeanu DF, Soucy E, Sato TF, Meister M, Murthy VN (2008) LED arrays as cost effective and efficient light sources for widefield microscopy. PLoS One 3(5):e2146
Bachtel AD, Gray RA, Stohlman JM, Bourgeois EB, Pollard AE, Rogers JM (2011) A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with di-4-ANEPPS using pulsed LED excitation. IEEE Trans Biomed Eng 58(7):2120–2126
Bernardinelli Y, Haeberli C, Chatton JY (2005) Flash photolysis using a light emitting diode: an efficient, compact, and affordable solution. Cell Calcium 37(6):565–572
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415(6868):198–205
Bray MA, Lin SF, Wikswo JP Jr (2000) Three-dimensional surface reconstruction and fluorescent visualization of cardiac activation. IEEE Trans Biomed Eng 47(10):1382–1391
Charney R, Cohen M (1993) The role of the coronary collateral circulation in limiting myocardial ischemia and infarct size. Am Heart J 126(4):937–945
Choi BR, Salama G (2000) Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol 529(Pt 1):171–188
Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J (1992) Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 355(6358):349–351
Efimov IR, Nikolski VP, Salama G (2004) Optical imaging of the heart. Circ Res 95(1):21–33
Ellis-Davies GC (2008) Neurobiology with caged calcium. Chem Rev 108(5):1603–1613
Entcheva E, Kostov Y, Tchernev E, Tung L (2004) Fluorescence imaging of electrical activity in cardiac cells using an all-solid-state system. IEEE Trans Biomed Eng 51(2):333–341
Evertson DW, Holcomb MR, Eames MC, Bray MA, Sidorov VY, Xu J, Wingard H, Dobrovolny HM, Woods MC, Gauthier DJ, Wikswo JP (2008) High-resolution high-speed panoramic cardiac imaging system. IEEE Trans Biomed Eng 55(3):1241–1243
Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Hear Rhythm 4(5):619–626
Herron TJ, Lee P, Jalife J (2012) Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 110(4):609–623
Holcomb MR, Woods MC, Uzelac I, Wikswo JP, Gilligan JM, Sidorov VY (2009) The potential of dual camera systems for multimodal imaging of cardiac electrophysiology and metabolism. Exp Biol Med (Maywood) 234(11):1355–1373
Hong M, Ross WN (2007) Priming of intracellular calcium stores in rat CA1 pyramidal neurons. J Physiol 584(Pt 1):75–87
Jia YZ, Sato S (1997) Evaluation of coronary collateral circulation in early ischemia in rat hearts. A morphological study. Nihon Ika Daigaku Zasshi 64(4):329–336
Jin L, Millard AC, Wuskell JP, Clark HA, Loew LM (2005) Cholesterol-enriched lipid domains can be visualized by di-4-ANEPPDHQ with linear and nonlinear optics. Biophys J 89(1):L04–L06
Jin L, Millard AC, Wuskell JP, Dong X, Wu D, Clark HA, Loew LM (2006) Characterization and application of a new optical probe for membrane lipid domains. Biophys J 90(7):2563–2575
Kay MW, Amison PM, Rogers JM (2004) Three-dimensional surface reconstruction and panoramic optical mapping of large hearts. IEEE Trans Biomed Eng 51(7):1219–1229
Kay M, Swift L, Martell B, Arutunyan A, Sarvazyan N (2008) Locations of ectopic beats coincide with spatial gradients of NADH in a regional model of low-flow reperfusion. Am J Physiol Heart Circ Physiol 294(5):H2400–H2405
Kornyeyev D, Reyes M, Escobar AL (2010) Luminal Ca(2+) content regulates intracellular Ca(2+) release in subepicardial myocytes of intact beating mouse hearts: effect of exogenous buffers. Am J Physiol Heart Circ Physiol 298(6):H2138–H2153
Lang D, Sulkin M, Lou Q, Efimov IR (2011) Optical Mapping of Action Potentials and Calcium Transients in the Mouse Heart. J Vis Exp (55):e3275. doi:10.3791/3275
Laurita KR, Singal A (2001) Mapping action potentials and calcium transients simultaneously from the intact heart. Am J Physiol Heart Circ Physiol 280(5):H2053–H2060
Lee P, Bollensdorff C, Quinn TA, Wuskell JP, Loew LM, Kohl P (2011) Single-sensor system for spatially resolved, continuous, and multiparametric optical mapping of cardiac tissue. Hear Rhythm 8(9):1482–1491
Lin SF, Wikswo JP (1999) Panoramic optical imaging of electrical propagation in isolated heart. J Biomed Opt 4(4):200–207
Lipp P, Luscher C, Niggli E (1996) Photolysis of caged compounds characterized by ratiometric confocal microscopy: a new approach to homogeneously control and measure the calcium concentration in cardiac myocytes. Cell Calcium 19(3):255–266
Lou Q, Li W, Efimov IR (2011) Multiparametric optical mapping of the Langendorff-perfused rabbit heart. J Vis Exp (55). doi:10.3791/3160
Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM (2007) Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Hear Rhythm 4(11):1441–1451
Qu F, Ripplinger CM, Nikolski VP, Grimm C, Efimov IR (2007) Three-dimensional panoramic imaging of cardiac arrhythmias in rabbit heart. J Biomed Opt 12(4):044019
Salama G, Hwang SM (2009) Simultaneous optical mapping of intracellular free calcium and action potentials from Langendorff perfused hearts. Curr Protoc Cytom. Jul;Chapter 12:Unit 12.17
Salama G, Lombardi R, Elson J (1987) Maps of optical action potentials and NADH fluorescence in intact working hearts. Am J Physiol 252(2 Pt 2):H384–H394
Sasaki T, Matsuki N, Ikegaya Y (2011) Action-potential modulation during axonal conduction. Science 331(6017):599–601
Scull JA, McSpadden LC, Himel HDt, Badie N, Bursac N (2011) Single-Detector Simultaneous Optical Mapping of V (m) and [Ca (2+)] (i) in Cardiac Monolayers. Ann Biomed Eng 40(5):1006–1017
Sobie EA, Kao JP, Lederer WJ (2007) Novel approach to real-time flash photolysis and confocal [Ca2+] imaging. Pflugers Arch 454(4):663–673
Swift L, Martell B, Khatri V, Arutunyan A, Sarvazyan N, Kay M (2008) Controlled regional hypoperfusion in Langendorff heart preparations. Physiol Meas 29(2):269–279
Waggoner AS, Wachman ES, Farkas DL (2001) Optical filtering systems for wavelength selection in light microscopy. Curr Protoc Cytom. 2001 May;Chapter 2:Unit 2.4
Witkowski FX, Leon LJ, Penkoske PA, Giles WR, Spano ML, Ditto WL, Winfree AT (1998) Spatiotemporal evolution of ventricular fibrillation. Nature 392(6671):78–82
Acknowledgements
This study was supported by the British Heart Foundation (P.K. and C.B.), the NIH grant R01EB001963 (P.Y. and L.M.L.) and the Human Frontier Science Program grant RGP0027/2009 (L.M.L.). We further gratefully acknowledge the University of Oxford Clarendon Fund Scholarship (P.L.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Peter Lee and Ping Yan are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOC 869 kb)
(MPEG 4692 kb)
(MPEG 3998 kb)
(MPEG 3714 kb)
(MPEG 3816 kb)
(MPEG 3642 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lee, P., Yan, P., Ewart, P. et al. Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques. Pflugers Arch - Eur J Physiol 464, 403–414 (2012). https://doi.org/10.1007/s00424-012-1135-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1135-6