Abstract
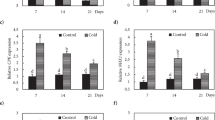
Bryophyte species growing in areas in which temperatures fall below zero in winter are likely to have tolerance to freezing stress. It is well established in higher plants that freezing tolerance is acquired by exposure to non-freezing low temperatures, accompanied by expression of various genes and increases in levels of the stress hormone abscisic acid (ABA). However, little is known about the physiological changes induced by cold acclimation in non-vascular plants such as bryophytes. We examined the effects of low temperatures on protonema cells of the moss Physcomitrella patens (Hedw.) Bruch & Schimp. The freezing tolerance of protonema cells was clearly increased by incubation at low temperatures ranging from 10°C to 0°C, with maximum tolerance achieved by incubation at 0°C for several days. The enhancement of freezing tolerance by low temperatures occurred in both light and dark conditions and was accompanied by accumulation of several transcripts for late-embryogenesis-abundant (LEA) proteins and boiling-soluble proteins. By de-acclimation, low-temperature-induced expression of these transcripts and proteins, as well as the freezing tolerance, was reduced. Interestingly, endogenous levels of ABA in tissues or that secreted into the culture medium were not specifically increased by low-temperature treatment. Furthermore, removal of ABA from the medium by addition of activated charcoal did not affect low-temperature-induced freezing tolerance of the protonema cells. Our results provide evidence that bryophytes have an ABA-independent cold-signaling pathway leading to expression of stress-related genes and resultant acquisition of freezing tolerance.







Similar content being viewed by others
Abbreviations
- ABA :
-
Abscisic acid
- GC–MS :
-
Gas chromatography–mass spectrometry
- LEA :
-
Late embryogenesis abundant
References
Anderson MD, Prasad TK, Martin BA, Stewart CR (1994) Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance. Plant Physiol 105:331–339
Asami T, Sekimata K, Wang JM, Yoneyama K, Takeuchi Y, Yoshida S (1999) Preparation of (±)-[1,2-13C2]abscisic acid for use as a stable and pure internal standard. J Chem Res [Suppl] 11:658–659
Bravo LA, Zúñiga GE, Alberdi M, Corcuera LJ (1998) The role of ABA in freezing tolerance and cold acclimation in barley. Physiol Plant 103:17–23
Burch J (2003) Some mosses survive cryopreservation without prior pretreatment. Bryologist 106:270–277
Chen HH, Li PH, Brenner ML (1983) Involvement of abscisic acid in potato cold acclimation. Plant Physiol 71:362–365
Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, Koshiba T, Sheen J (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14:2723–2743
Close TJ (1997) Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol Plant 100:291–296
Daie J, Campbell WF (1981) Response of tomato plants to stressful temperatures. Plant Physiol 67:26–29
Fellner M, Zhang R, Pharis RP, Sawhney VK (2001) Reduced de-etiolation of hypocotyl growth in a tomato mutant is associated with hypersensitivity to, and high endogenous levels of, abscisic acid. J Exp Bot 52:725–738
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124:1854–1865
Gray GR, Chauvin LP, Sarhan F, Huner NPA (1997) Cold acclimation and freezing tolerance (a complex interaction of light and temperature). Plant Physiol 114:467–474
Hara M, Terashima S, Fukaya T, Kuboi (2003) Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dihedron in transgenic tobacco. Planta 217:290–298
Heino P, Sandman G, Lång V, Nordin K, Palva ET (1990) Abscisic acid deficiency prevents development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 79:801–806
Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9:1935–1949
Jagglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O. Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280:104–106
Jouve L, Franck T, Gaspar T, Cattivelli L, Hausman JF (2000) Poplar acclimation to cold during in vitro conservation at low non-freezing temperature: metabolic and proteic changes. J Plant Physiol 157:117–123
Kim HJ, Kim YK, Park JY, Kim J (2002) Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J 29:693–704
Kobayashi F, Takumi S, Nakata M, Ohno R, Nakamura T, Nakamura C (2004) Comparative study of the expression profiles of the Cor/Lea gene family in two wheat cultivars with contrasting levels of freezing tolerance. Physiol Plant 120:585–594
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lalk I, Dörffling K (1985) Hardening, abscisic acid, proline and freezing resistance in two winter wheat varieties. Physiol Plant 63:287–292
Lång V, Mäntylä E, Welin B, Sundberg B, Palva ET (1994) Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol 104:1341–1349
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199–222
Levitt J (1980) Responses of plants to environmental stresses, vol 1. Academic Press, New York
Li C, Puhakainen T, Welling A, Viherä-Aarnio A, Ernstsen A, Junttila O, Heino P, Palva ET (2002) Cold acclimation in silver birch (Betula pendula). Development of freezing tolerance in different tissues and climatic ecotypes. Physiol Plant 116:478–488
Lin C, Guo WW, Everson E, Thomashow MF (1990) Cold acclimation in Arabidopsis and wheat. A response associated with expression of related genes encoding ‘boiling-stable’ polypeptides. Plant Physiol 94:1078–1083
Loik M, Nobel PS (1993) Exogenous abscisic acid mimics cold acclimation for cacti differing in freezing tolerance. Plant Physiol 103:871–876
Machuka J, Bashiardes S, Ruben E, Spooner K, Cuming A, Knight C, Cove D (1999) Sequence analysis of expressed sequence tags from an ABA-treated cDNA library identifies stress response genes in the moss Physcomitrella patens. Plant Cell Physiol 40:378–387
Minami A, Nagao M, Arakawa K, Fujikawa S, Takezawa D (2003) Abscisic acid-induced freezing tolerance in the moss Physcomitrella patens is accompanied by increased expression of stress-related genes. J Plant Physiol 160:475–483
NDong C, Danyluk J, Wilson KE, Pocock T, Huner NPA, Sarhan F (2002) Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses. Plant Physiol 129:1368–1381
Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, Kohara Y, Hasebe M (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100:8007–8012
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Oliver MJ, Tuba Z, Mishler BD (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151:85–100
Pearce RS (1999) Molecular analysis of acclimation to cold. Plant Growth Regul 29:47–76
Pengelly WL (1986) Validation of immunoassays. In: Bopp M (ed) Plant growth substances 1985. Springer, Berlin Heidelberg New York, pp 35–43
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytol 156:327–349
Reutter K, Atzorn R, Hadeler B, Schmülling T, Reski R (1998) Expression of the bacterial ipt gene in Physcomitrella rescues mutations in budding and in plastid division. Planta 206:196–203
Robinson SA, Wasley J, Tobin AK (2003) Living on the edge—plants and global change in continental and maritime Antarctica. Global Change Biol 9:1681–1717
Rütten D, Santarius KA (1992) Relationship between frost tolerance and sugar concentration of various bryophytes in summer and winter. Oecologia 91:260–265
Ryu SB, Li PH (1994) Potato cold hardiness development and abscisic acid. II. De novo synthesis of proteins is required for the increase in free abscisic acid during potato (Solanum commersonii) cold acclimation. Physiol Plant 90:21–26
Sambrook J, Fritisch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schaefer DG (2002) A new moss genetics: targeted mutagenesis in Physcomitrella patens. Annu Rev Plant Biol 53:477–501
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13:61–72
Steponkus PL (1971) Cold acclimation of Hedera helix (evidence for a two phase process). Plant Physiol 47:175–180
Takezawa D, Minami A (2004) Calmodulin-binding proteins in bryophytes: identification of abscisic acid-, cold-, and osmotic stress-induced genes encoding novel membrane-bound transporter-like proteins. Biochem Biophys Res Commun 317:428–436
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Uemura M, Warren G, Steponkus PL (2003) Freezing sensitivity in the sfr4 mutant of Arabidopsis is due to low sugar content and is manifested by loss of osmotic responsiveness. Plant Physiol 131:1800–1807
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wanner LA, Junttila O (1999) Cold-induced freezing tolerance in Arabidopsis. Plant Physiol 120:391–399
Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM (1996) The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol 111:363–370
Werner O, Ros Espin RM, Bopp M, Atzorn R (1991) Abscisic-acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186:99–103
Wise MJ (2003) LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52
Wise MJ, Tunnacliffe A (2004) POPP the question: What do LEA proteins do? Trends Plant Sci 9:13–17
Xin Z, Browse J (2000) Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell Environ 23:893–902
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell [Suppl] 14:165–183
Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Acknowledgements
We thank Takako Matsushita for her technical assistance. We also thank Dr. Asami at the Institute of Physical and Chemical Research (RIKEN), Japan for providing the [13C2]ABA. This work was supported by a grant-in aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and “Probrain” from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minami, A., Nagao, M., Ikegami, K. et al. Cold acclimation in bryophytes: low-temperature-induced freezing tolerance in Physcomitrella patens is associated with increases in expression levels of stress-related genes but not with increase in level of endogenous abscisic acid. Planta 220, 414–423 (2005). https://doi.org/10.1007/s00425-004-1361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1361-z