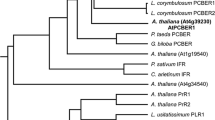
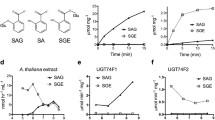
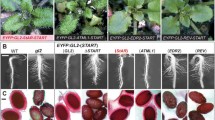
Abstract
Mammalian sulfotransferases (EC 2.8.2) are involved in many important facets of steroid hormone activity and metabolism. In this study, Arabidopsis AtST4a and AtST1 were identified and characterized as brassinosteroid sulfotransferases that appear to be involved in different aspects of hormone regulation. The two proteins share 44% identity in amino acid sequence, and belong to different plant sulfotransferase families. AtST4a was specific for biologically active end products of the brassinosteroid pathway. The enzyme sulfated brassinosteroids with diverse side-chain structures, including 24-epibrassinosteroids and the naturally occurring (22R, 23R)-28-homobrassinosteroids. AtST4a belongs to a small subfamily of sulfotransferases having two other members, AtST4b and -c. Among the three recombinant enzymes, only AtST4a was catalytically active with brassinosteroids. Transcript expression of AtST4 subfamily members was largely specific to the root. AtST4b- and -c transcript levels were induced by treatment with trans-zeatin, while AtST4a was repressed under the same conditions, supporting a divergent function of AtST4a. Co-regulation of AtST4b and -c correlated with their location in tandem on chromosome 1. AtST1 was stereospecific for 24-epibrassinosteroids, with a substrate preference for the metabolic precursor 24-epicathasterone, and exhibited catalytic activity with hydroxysteroids and estrogens. To gain more insight into this dual activity with plant and mammalian steroids, enzymatic activities of human steroid sulfotransferases toward brassinosteroids were characterized. The dehydroepiandrosterone sulfotransferase SULT2A1 displayed catalytic activity with a selected set of 24-epibrassinolide precursors, including 24-epicathasterone, with specific activities comparable to that measured for the endogenous substrate dehydroepiandrosterone. The comparable activity profiles of AtST1 and SULT2A1 suggest a similar architecture of the acceptor-binding site between the two enzymes, and may potentially reflect a common ability to conjugate certain xenobiotics.




Similar content being viewed by others
Abbreviations
- TAIR:
-
The arabidopsis information resource
- IPTG:
-
Isopropyl β-d-thiogalactopyranoside
- PAPS:
-
3′-Phosphoadenosine 5′-phosphosulfate
- DON:
-
Deoxynivalenol
References
Adam G, Schneider B (1999) Uptake, transport and metabolism. In: Sakurai A, Yokota T, Clouse SD (eds) Brassinosteroids: steroidal plant hormones. Springer-Verlag, Tokyo, pp 113–136
Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002) Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130:504–513
Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW (2004) A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics 14:199–211
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brenner WG, Romanov GA, Kollmer I, Burkle L, Schmulling T (2005) Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J 44:314–333
Castle J, Szekeres M, Jenkins G, Bishop GJ (2005) Unique and overlapping expression patterns of CYP85 genes involved in brassinosteroid C-6 oxidation. Plant Mol Biol:129–140
Chang HJ, Zhou M, Lin SX (2001) Human dehydroepiandrosterone sulfotransferase: purification and characterization of a recombinant protein. J Steroid Biochem Mol Biol 77:159–165
Chang HJ, Shi R, Rehse P, Lin SX (2004) Identifying androsterone (ADT) as a cognate substrate for human dehydroepiandrosterone sulfotransferase (DHEA-ST) important for steroid homeostasis. J Biol Chem 279:2689–2696
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Ann Rev Plant Physiol Plant Mol Biol 49:427–451
D’Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124:1706–1717
Falany CN (1997) Enzymology of human cytosolic sulfotransferases. FASEB J 11:206–216
Falany CN, Vazquez ME, Kalb JM (1989) Purification and characterization of human liver dehydroepiandrosterone sulphotransferase. Biochem J 260:641–646
Falany CN, Krasnykh V, Falany JL (1995) Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol 52:529–539
Fridman E, Pichersky E (2005) Metabolomics, genomics, proteomics, and the identification of enzymes and their substrates and products. Curr Opin Plant Biol 8:242–248
Gamage NU, Tsvetanov S, Duggleby RG, McManus ME, Martin JL (2005) The structure of human SULT1A1 crystallized with estradiol. J Biol Chem 280:41482–41486
Gidda SK, Miersch O, Levitin A, Schmidt J, Wasternack C, Varin L (2003) Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J Biol Chem 278:17895–17900
Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, Pabel U, Pomplun D, Teubner W, Meinl W (2001) Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat Res 482:27–40
Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130:1319–1334
Hempel N, Barnett AC, Bolton-Grob RM, Liyou NE, McManus ME (2000) Site-directed mutagenesis of the substrate-binding cleft of human estrogen sulfotransferase. Biochem Biophys Res Commun 276:224–230
Hirai MY, Klein M, Fujikawa Y, Yano M, Goodenowe DB, Yamazaki Y, Kanaya S, Nakamura Y, Kitayama M, Suzuki H, Sakurai N, Shibata D, Tokuhisa J, Reichelt M, Gershenzon J, Papenbrock J, Saito K (2005) Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J Biol Chem 280:25590–25595
Hobkirk R (1985) Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol 63:1127–1144
Kim HB, Kwon M, Ryu H, Fujioka S, Takatsuto S, Yoshida S, An CS, Lee I, Hwang I, Choe S (2006) The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiol 140:548–557
Kim TW, Hwang JY, Kim YS, Joo SH, Chang SC, Lee JS, Takatsuto S, Kim SK (2005) Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 17:2397–2412
Kim YS, Kim TW, Kim SK (2005) Brassinosteroids are inherently biosynthesized in the primary roots of maize, Zea mays L. Phytochemistry 66:1000–1006
Klein M, Papenbrock J (2004) The multi-protein family of Arabidopsis sulphotransferases and their relatives in other plant species. J Exp Bot 55:1809–1820
Klein M, Reichelt M, Gershenzon J, Papenbrock J (2006) The three desulfoglucosinolate sulfotransferase proteins in Arabidopsis have different substrate specificities and are differentially expressed. FEBS J 273:122–136
Lacomme C, Roby D (1996) Molecular cloning of a sulfotransferase in Arabidopsis thaliana and regulation during development and in response to infection with pathogenic bacteria. Plant Mol Biol 30:995–1008
Laemlli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li J, Biswas MG, Chao A, Russell DW, Chory J (1997) Conservation of function between mammalian and plant steroid 5α–reductases. Proc Natl Acad Sci USA 94:3554–3559
Luu-The V, Dufort I, Paquet N, Reimnitz G, Labrie F (1995) Structural characterization and expression of the human dehydroepiandrosterone sulfotransferase gene. DNA Cell Biol 14:511–518
Manabe Y, Miki B (2006) How imidazolinone kills plants—the transcriptome profiling of csr1-2 upon imidazolinone herbicide treatment. Abstract of the 17th international conference on arabidopsis research. Madison, WI
Marsolais F, Varin L (1995) Identification of amino acid residues critical for catalysis and cosubstrate binding in the flavonol 3-sulfotransferase. J Biol Chem 270:30458–30463
Marsolais F, Gidda SK, Boyd J, Varin L (2000) Plant soluble sulfotransferases: structural and functional similarity with mammalian enzymes. Rec Adv Phytochem 34:433–456
Marsolais F, Sebastia CH, Rousseau A, Varin L (2004) Molecular and biochemical characterization of BNST4, an ethanol-inducible steroid sulfotransferase from Brassica napus, and regulation of BNST genes by chemical stress and during development. Plant Sci 166:1359–1370
Meloche CA, Sharma V, Swedmark S, Andersson P, Falany CN (2002) Sulfation of budesonide by human cytosolic sulfotransferase, dehydroepianderosterone-sulfotransferase (DHEA-ST). Drug Metab Dispos 30:582–585
Nakamura M, Satoh T, Tanaka SI, Mochizuki N, Yokota T, Nagatani A (2005) Activation of the cytochrome P450 gene, CYP72C1, reduces the levels of active brassinosteroids in vivo. J Exp Bot 56:833–840
Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J (1999) BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA 96:15316–15323
Nomura T, Kushiro T, Yokota T, Kamiya Y, Bishop GJ, Yamaguchi S (2005) The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J Biol Chem 280:17873–17879
Piotrowski M, Schemenewitz A, Lopukhina A, Muller A, Janowitz T, Weiler EW, Oecking C (2004) Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J Biol Chem 279:50717–50725
Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G (2003) Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem 278:47905–47914
Poppenberger B, Fujioka S, Soeno K, George GL, Vaistij FE, Hiranuma S, Seto H, Takatsuto S, Adam G, Yoshida S, Bowles D (2005) The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proc Natl Acad Sci USA 102:15253–15258
Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC (2001) Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142:5342–5350
Rouleau M, Marsolais F, Richard M, Nicolle L, Voigt B, Adam G, Varin L (1999) Inactivation of brassinosteroid biological activity by a salicylate-inducible steroid sulfotransferase from Brassica napus. J Biol Chem 274:20925–20930
Runge-Morris M, Kocarek TA (2005) Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab 6:299–307
Shibutani S, Shaw PM, Suzuki N, Dasaradhi L, Duffel MW, Terashima I (1998) Sulfation of alpha-hydroxytamoxifen catalyzed by human hydroxysteroid sulfotransferase results in tamoxifen-DNA adducts. Carcinogenesis 19:2007–2011
Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop GJ, Yoshida S (2001) Brassinosteroid 6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol 126:770–779
Shimada Y, Goda H, Nakamura A, Takatsuto S, Fujioka S, Yoshida S (2003) Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol 131:287–297
Smith DW, Johnson KA, Bingman CA, Aceti DJ, Blommel PG, Wrobel RL, Frederick RO, Zhao Q, Sreenath H, Fox BG, Volkman BF, Jeon WB, Newman CS, Ulrich EL, Hegeman AD, Kimball T, Thao S, Sussman MR, Markley JL, Phillips GN (2004) Crystal structure of At2g03760, a putative steroid sulfotransferase from Arabidopsis thaliana. Proteins 57:854–857
Strott CA (2002) Sulfonation and molecular action. Endocr Rev 23:703–732
Symons GM, Reid JB (2004) Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiol 135:2196–2206
Takahashi N, Nakazawa M, Shibata K, Yokota T, Ishikawa A, Suzuki K, Kawashima M, Ichikawa T, Shimada H, Matsui M (2005) Shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. Plant J 42:13–22
Takatsuto S, Yokota T (1999) Biochemical analysis of natural brassinosteroids. In: Sakurai A, Yokota T, Clouse SD (eds) Brassinosteroids: steroidal plant hormones. Springer-Verlag, Tokyo, pp 47–68
Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC (2005) Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med 11:153–159
Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Wang HC, Torres QI, Ward JM, Murthy G, Zhang JY, Walker JC, Neff MM (2005) Bas1 and Sob7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J 42:23–34
Varin L, Barron D, Ibrahim RK (1987) Enzymatic assay for flavonoid sulfotransferase. Anal Biochem 161:176–180
Varin L, Ibrahim RK (1992) Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence. J Biol Chem 267:1858–1863
Varin L, DeLuca V, Ibrahim RK, Brisson N (1992) Molecular characterization of two plant flavonol sulfotransferases. Proc Natl Acad Sci USA 89:1286–1290
Winter J, Schneider B, Strack D, Adam G (1997) Role of a cytochrome P450-dependent monooxygenase in the hydroxylation of 24-epi-brassinolide. Phytochemistry 45:233–237
Yokota T, Ogino Y, Suzuki H, Takahashi N, Saimoto H, Fujioka S, Sakurai A (1991) Metabolism and biosynthesis of brassinosteroids. ACS Symp Ser 474:86–96
Yokota T (1997) The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 2:137–143
Yokota T, Higuchi K, Kosaka Y, Takahashi N (1992) Transport and metabolism of brassinosteroids in rice. In: Karssen CM, Van Loon LC, Vreugdenhil D (eds) Progress in plant growth regulation. Kluwer Academic Publishers, Dordrecht, pp 298–305
Acknowledgments
We thank Dr. G. Adam (Leibniz Institute of Plant Biochemistry, Halle, Germany) for brassinosteroid substrates, Dr. D. Roby (Centre National de la Recherche Scientifique/Institut National de la Recherche Agronomique, Castanet-Tolosan, France) for the gift of the AtST1 cDNA (RaR047), Dr. C. N. Falany (University of Alabama at Birmingham, AL, USA) for the gift of the human SULT1E1 cDNA, Dr. V. Luu-The (Centre de recherche du CHUL, Sainte-Foy, QU, Canada) for the gift of the human SULT2A1 cDNA, and Drs. Yuzuki Manabe and Brian Miki (Agriculture and Agri-Food Canada, Eastern Cereal and Oilseed Research Centre, Ottawa, ON, Canada) for sharing data prior to publication. We also thank Alex Molnar for assistance in preparing the figures. F. M. was supported in part by a postgraduate scholarship from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and a Concordia J. W. McConnell Memorial graduate fellowship. This work was supported by NSERC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marsolais, F., Boyd, J., Paredes, Y. et al. Molecular and biochemical characterization of two brassinosteroid sulfotransferases from Arabidopsis, AtST4a (At2g14920) and AtST1 (At2g03760). Planta 225, 1233–1244 (2007). https://doi.org/10.1007/s00425-006-0413-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0413-y