Abstract
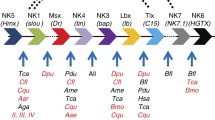
A number of examples of independently duplicated regulatory genes have been identified in cnidarians, but the extent of this phenomenon and organization of these duplicated genes are unknown. Here we describe the identification of three pairs of independently duplicated homeobox genes in the anthozoan cnidarian, Acropora millepora. In each case, the pairs of paralogous genes are tightly linked, but the extent of sequence divergence implies that these do not reflect recent duplication events. The phenomenon is likely to be more general, as the examples reported here represent most of the limited number of Acropora homeobox genes for which genomic data are yet available.



Similar content being viewed by others
References
Adachi J, Hasegawa M (1996) MOLPHY version 2.3: programs for molecular phylogenetics based on maximum likelihood. Comput Sci Monogr 28:1–150
Bridge DM, Stover NA, Steele RE (2000) Expression of a novel receptor tyrosine kinase gene and a paired-like homeobox gene provides evidence of differences in patterning at the oral and aboral ends of hydra. Dev Biol 220:253–262. DOI 10.1006/dbio.2000.9653
Broccoli V, Colombo E, Cossu G (2002) Dmbx1 is a paired-box containing gene specifically expressed in the caudal most brain structures. Mech Dev 114:219–223. DOI 10.1016/S0925-4773(02)00078-3
Chen JY, Oliveri P, Gao F, Dornbos SQ, Li CW, Bottjer DJ, Davidson EH (2002) Precambrian animal life: probable developmental and adult cnidarian forms from Southwest China. Dev Biol 248:182–196. DOI 10.1006/dbio.2002.0714
Conway Morris S (2000) The Cambrian “explosion”: slow-fuse or megatonnage? Proc Natl Acad Sci USA 97:4426–4429
Cowden J, Levine M (2003) Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev Biol 262:335–349. DOI 10.1016/S0012-1606(03)00395-6
Furlong R, Holland P (2002) Were vertebrates octoploid? Philos Trans R Soc Lond B Biol Sci 357:531–544. DOI 10.1098/rstb.2001.1035
Galliot B, de Vargas C, Miller D (1999) Evolution of homeobox genes: Q50 paired-like genes founded the paired class. Dev Genes Evol 209:186–197. DOI 10.1007/s004270050243
Gauchat D, Kreger S, Holstein T, Galliot B (1998) Prdl-a, a gene marker for hydra apical differentiation related to triploblastic paired-like head-specific genes. Development 125:1637–1645
Gauchat D, Mazet F, Berney C, Schummer M, Kreger S, Pawlowski J, Galliot, B (2000) Evolution of Antp-class genes and differential expression of Hydra Hox/paraHox genes in anterior patterning. Proc Natl Acad Sci USA 97:4493–4498
Gogoi RN, Schubert FR, Martinez-Barbera JP, Acampora D, Simeone A, Lumsden A (2002) The paired-type homeobox gene Dmbx1 marks the midbrain and pretectum. Mech Dev 114:213–217. DOI 10.1016/S0925-4773(02)00067-9
Grasso LC, Hayward DC, Trueman JW, Hardie KM, Janssens PA, Ball EE (2001) The evolution of nuclear receptors: evidence from the coral Acropora. Mol Phylogenet Evol 21:93–102. DOI 10.1006/mpev.2001.0994
Hovde S, Abate-Shen C, Geiger J (2001) Crystal structure of the Msx-1 homeodomain/DNA complex. Biochemistry 40:12013–12021. DOI 10.1021/bi0108148
Kawahara A, Chien CB, Dawid IB (2002) The homeobox gene mbx is involved in eye and tectum development. Dev Biol 248:107–117. DOI 10.1006/dbio.2002.0709
Kortschak RD, Samuel G, Saint R, Miller DJ (2003) EST analysis of the cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Curr Biol 13:2190–2195. DOI 10.1016/j.cub.2003.11.030
Martindale MQ, Pang K, Finnerty JR (2004) Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131:2463–2474. DOI 10.1242/dev.01119
Martinez-Barbera JP, Signore M, Boyl PP, Puelles E, Acampora D, Gogoi R, Schubert F, Lumsden A, Simeone A (2001) Regionalisation of anterior neuroectoderm and its competence in responding to forebrain and midbrain inducing activities depend on mutual antagonism between OTX2 and GBX2. Development 128:4789–4800
Minguillon C, Ferrier DE, Cebrian C, Garcia-Fernandez J (2002) Gene duplications in the prototypical cephalochordate amphioxus. Gene 287:121–128. DOI 10.1016/S0378-1119(01)00828-9
Mochizuki K, Sano H, Kobayashi S, Nishimiya-Fujisawa C, Fujisawa T (2000) Expression and evolutionary conservation of nanos-related genes in Hydra. Dev Genes Evol 210:591–602. DOI 10.1007/s004270000105
Ohtoshi A, Nishijima I, Justice MJ, Behringer RR (2002) Dmbx1, a novel evolutionarily conserved paired-like homeobox gene expressed in the brain of mouse embryos. Mech Dev 110:241–244. DOI 10.1016/S0925-4773(01)00587-1
Samuel G, Miller D, Saint R (2001) Conservation of a DPP/BMP signaling pathway in the nonbilateral cnidarian Acropora millepora. Evol Dev 3:241–250
Schummer M, Scheurlen I, Schaller C, Galliot B (1992) HOM/HOX homeobox genes are present in hydra (Chlorohydra viridissima) and are differentially expressed during regeneration. EMBO J 11:1815–1823
Smith S, Jaynes J (1996) A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2- and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development 122:3141–3150
Takahashi T, Holland PW, Cohn MJ, Shimizu K, Kurokawa M, Hirai H (2002) An orphan PRD class homeobox gene expressed in mouse brain and limb development. Dev Genes Evol 212:293–297. DOI 10.1007/s00427-002-0244-1
Wang C, Brodnicki T, Copeland N, Jenkins N, Harvey R (2000) Conserved linkage of NK-2 homeobox gene pairs Nkx2-2/2-4 and Nkx2-1/2-9 in mammals. Mamm Genome 11:466–468
Watada H, Mirmira R, Kalamaras J, German M (2000) Intramolecular control of transcriptional activity by the NK2-specific domain in Nk-2 homeodomain proteins. Proc Natl Acad Sci USA 97:9443–9448
Zhang Y, Miki T, Iwanaga T, Koseki Y, Okuno M, Sunaga Y, Ozaki N, Yano H, Koseki H, Seino S (2002) Identification, tissue expression, and functional characterization of Otx3, a novel member of the Otx family. J Biol Chem 277:28065–29069. DOI 10.1074/jbc.C100767200
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Hislop and D. de Jong contributed equally to this work
Edited by M. Akam
Rights and permissions
About this article
Cite this article
Hislop, N.R., de Jong, D., Hayward, D.C. et al. Tandem organization of independently duplicated homeobox genes in the basal cnidarian Acropora millepora. Dev Genes Evol 215, 268–273 (2005). https://doi.org/10.1007/s00427-005-0468-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-005-0468-y