Abstract
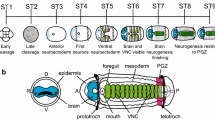
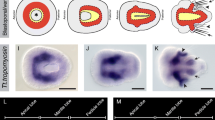
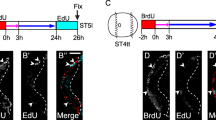
To investigate the evolutionary history of mesoderm in the bilaterian lineage, we are studying mesoderm development in the polychaete annelid, Capitella sp. I, a representative lophotrochozoan. In this study, we focus on the Twist and Snail families as candidate mesodermal patterning genes and report the isolation and in situ expression patterns of two twist homologs (CapI-twt1 and CapI-twt2) and two snail homologs (CapI-sna1 and CapI-sna2) in Capitella sp. I. CapI-twt1 is expressed in a subset of mesoderm derivatives during larval development, while CapI-twt2 shows more general mesoderm expression at the same stages. Neither twist gene is detected before the completion of gastrulation. The two snail genes have very distinct expression patterns. At cleavage and early gastrula stages, CapI-sna1 is broadly expressed in precursors of all three germ layers and becomes restricted to cells around the closing blastopore during late gastrulation; CapI-sna2 expression is not detected at these stages. After gastrulation, both snail genes are expressed in the developing central nervous system (CNS) at stages when neural precursor cells are internalized, and CapI-sna1 is also expressed laterally within the segmental mesoderm. Based on the expression patterns in this study, we suggest a putative function for Capitella sp. I twist genes in mesoderm differentiation and for snail genes in regulating CNS development and general cell migration during gastrulation.





Similar content being viewed by others
References
Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105
Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M (1991) The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development 111:983–992
Anderson DT (1973) Embryology and phylogeny in Annelids and Arthropods. Pergamon, Oxford
Ashraf SI, Ip YT (2001) The Snail protein family regulates neuroblast expression of inscuteable and string, genes involved in asymmetry and cell division in Drosophila. Development 128:4757–4767
Ashraf SI, Hu X, Roote J, Ip YT (1999) The mesoderm determinant snail collaborates with related zinc-finger proteins to control Drosophila neurogenesis. EMBO J 18:6426–6438
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Baylies MK, Bate M (1996) twist: a myogenic switch in Drosophila. Science 272:1481–1484
Bhup R, Marsden JR (1981) The development of the central nervous system in Capitella capitata (Polychaeta, Annelida). Can J Zool 60:2284–2295
Cai Y, Chia W, Yang X (2001) A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J 20:1704–1714
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Castanon I, Baylies MK (2002) A Twist in fate: evolutionary comparison of Twist structure and function. Gene 287:11–22
Cripps RM, Black BL, Zhao B, Lien CL, Schulz RA, Olson EN (1998) The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev 12:422–434
Dohle W, Gerberding M, Hejnol A, Scholtz G (2004) Cell lineage, segment differentiation, and gene expression in crustaceans. In: Scholtz G (ed) Evolutionary developmental biology of Crustacea. Sweets and Zeitlinger, Lisse, The Netherlands, pp 95–133
Eckelbarger KJ, Linley PA, Grassle JP (1984) Role of ovarian follicle cells in vitellogenesis and oocyte resorption in Capitella sp. I (Polychaeta). Mar Biol 79:133–144
Eisig H (1899) Zur Entwicklungsgeschichte der Capitelliden. Mittheilungen Aus der Zoologischen Station Zu Neapel 13:5–39
Frobius AC, Seaver EC (2006) Capitella sp. I homeobrain-like, the first lophotrochozoan member of a novel paired-like homeobox gene family. Gene Expr Patterns 6:985–991
Gerberding M, Browne WE, Patel NH (2002) Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates. Development 129:5789–5801
Goldstein B, Leviten MW, Weisblat DA (2001) Dorsal and snail homologs in leech development. Dev Genes Evol 211:329–337
Grassle JP, Grassle JF (1976) Sibling species in the marine polution indicator Capitella (Polychaeta). Science 192:567–569
Grau Y, Carteret C, Simpson P (1984) Mutations and chromosomal rearrangements affecting the expression of snail, a gene involved in embryonic patternin in Drosophila melanogaster. Genetics 108:347–360
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Handel K, Basal A, Fan X, Roth S (2005) Tribolium castaneum twist: gastrulation and mesoderm formation in a short-germ beetle. Dev Genes Evol 215:13–31
Harfe BD, Vaz Gomes A, Kenyon C, Liu J, Krause M, Fire A (1998) Analysis of a Caenorhabditis elegans Twist homolog identifies conserved and divergent aspects of mesodermal patterning. Genes Dev 12:2623–2635
Hopwood ND, Pluck A, Gurdon JB (1989) A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell 59:893–903
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Langeland JA, Tomsa JM, Jackman WR Jr, Kimmel CB (1998) An amphioxus snail gene: expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Dev Genes Evol 208:569–577
Leptin M (1991) twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev 5:1568–1576
Lespinet O, Nederbragt AJ, Cassan M, Dictus WJ, van Loon AE, Adoutte A (2002) Characterisation of two snail genes in the gastropod mollusc Patella vulgata. Implications for understanding the ancestral function of the snail-related genes in Bilateria. Dev Genes Evol 212:186–195
Mayor R, Essex LJ, Bennett MF, Sargent MG (1993) Distinct elements of the xsna promoter are required for mesodermal and ectodermal expression. Development 119:661–671
Nederbragt AJ, Lespinet O, van Wageningen S, van Loon AE, Adoutte A, Dictus WJ (2002) A lophotrochozoan twist gene is expressed in the ectomesoderm of the gastropod mollusk Patella vulgata. Evol Dev 4:334–343
Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3:155–166
Nieto MA, Bennett MF, Sargent MG, Wilkinson DG (1992) Cloning and developmental expression of Sna, a murine homologue of the Drosophila snail gene. Development 116:227–237
Nieto MA, Sargent MG, Wilkinson DG, Cooke J (1994) Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264:835–839
Nusslein-Volhard C, Weischaus E, Kluding H (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilheim Roux Arch Dev Biol 193:267–282
Perez-Pomares JM, Munoz-Chapuli R (2002) Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec 268:343–351
Pioro HL, Stollewerk A (2006) The expression pattern of genes involved in early neurogenesis suggests distinct and conserved functions in the diplopod Glomeris marginata. Dev Genes Evol 216:417–430
Price AL, Patel NH (2007) Investigating divergent mechanisms of mesoderm development in arthropods: the expression of Ph-twist and Ph-mef2 in Parhyale hawaiensis. J Exp Zoolog B Mol Dev Evol (in press)
Ruppert E, Barnes R (1994) Invertebrate zoology. Thomson learning, Belmont
Sargent MG, Bennett MF (1990) Identification in Xenopus of a structural homologue of the Drosophila gene snail. Development 109:967–973
Saulnier-Michel C (1992) Polychaeta: Digestive system. Wiley-Liss, New York
Seaver EC, Kaneshige LM (2006) Expression of ‘segmentation’ genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Dev Biol 289:179–194
Seaver EC, Paulson DA, Irvine SQ, Martindale MQ (2001) The spatial and temporal expression of Ch-en, the engrailed gene in the polychaete Chaetopterus, does not support a role in body axis segmentation. Dev Biol 236:195–209
Seaver EC, Thamm K, Hill SD (2005) Growth patterns during segmentation in the two polychaete annelids, Capitella sp. I and Hydroides elegans: comparisons at distinct life history stages. Evol Dev 7:312–326
Simpson P (1983) Maternal-zygotic gene interactions during formation of the dorsoventral pattern in Drosophila embryos. Genetics 105:615–632
Smith DE, Franco del Amo F, Gridley T (1992) Isolation of Sna, a mouse gene homologous to the Drosophila genes snail and escargot: its expression pattern suggests multiple roles during postimplantation development. Development 116:1033–1039
Sommer RJ, Tautz D (1994) Expression patterns of twist and snail in Tribolium (Coleoptera) suggest a homologous formation of mesoderm in long and short germ band insects. Dev Genet 15:32–37
Swofford DL (2000) PAUP*: Phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland, MA
Tavares AT, Izpisuja-Belmonte JC, Rodriguez-Leon J (2001) Developmental expression of chick twist and its regulation during limb patterning. Int J Dev Biol 45:707–713
Technau U, Scholz CB (2003) Origin and evolution of endoderm and mesoderm. Int J Dev Biol 47:531–539
Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F (1988) Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J 7:2175–2183
Thisse C, Thisse B, Schilling TF, Postlethwait JH (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119:1203–1215
Thisse C, Thisse B, Postlethwait JH (1995) Expression of snail2, a second member of the zebrafish snail family, in cephalic mesendoderm and presumptive neural crest of wild-type and spadetail mutant embryos. Dev Biol 172:86–99
Wada S, Saiga H (1999) Cloning and embryonic expression of Hrsna, a snail family gene of the ascidian Halocynthia roretzi: implication in the origins of mechanisms for mesoderm specification and body axis formation in chordates. Dev Growth Differ 41:9–18
Wang SM, Coljee VW, Pignolo RJ, Rotenberg MO, Cristofalo VJ, Sierra F (1997) Cloning of the human twist gene: its expression is retained in adult mesodermally derived tissues. Gene 187:83–92
Weller M, Tautz D (2003) Prospero and Snail expression during spider neurogenesis. Dev Genes Evol 213:554–566
Werbrock AH, Meiklejohn DA, Sainz A, Iwasa JH, Savage RM (2001) A polychaete hunchback ortholog. Dev Biol 235:476–488
Wolf C, Thisse C, Stoetzel C, Thisse B, Gerlinger P, Perrin-Schmitt F (1991) The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev Biol 143:363–373
Yasui K, Zhang SC, Uemura M, Aizawa S, Ueki T (1998) Expression of a twist-related gene, Bbtwist, during the development of a lancelet species and its relation to cephalochordate anterior structures. Dev Biol 195:49–59
Acknowledgements
The Capitella sp. I genomic and EST sequence data were produced by the US Department of Energy Joint Genome Institute (http://www.jgi.doe.gov/). We thank lab members Néva Meyer and Michael Boyle for sharing unpublished data and commenting on this manuscript, and Lori Kaneshige for assistance with preliminary in situ experiments. We also thank David Matus for assistance with gene orthology analyses and two anonymous reviewers for insightful comments. This work was supported by NSF grants IOB05-44869 and EF0531558.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D.A. Weisblat
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(A) Predicted amino acid sequences of the basic helix-loop-helix domains and WR motifs of CapI-Twt1 and CapI-Twt2 aligned with the corresponding domains of Twist homologs from other species. Consensus residues (as defined in Castanon and Baylies, 2002) for the ‘twist type’ basic helix-loop-helix domain and for the WR motif are shown in the bottom line of the alignment. (B) Predicted amino acid sequences of the 5 zinc-finger domains of CapI-Sna1 and CapI-Sna2 and the SNAG domain of CapI-Sna1 aligned with the corresponding domains of Snail homologs from other species. The first zinc-finger domain is not present in the human protein (Hs-sna) and the SNAG domain is not present in CapI-Sna2. Snail consensus residues in the zinc-finger sequences are shown in the bottom line of the alignment, shaded residues in CapI-Sna1 and CapI-Sna2 sequences indicate residues that differ from the conserved sequence. In each alignment, amino acid identities are indicated as dashes. Species abbreviations are as follows: CapI, Capitella sp. I; Pv, Patella vulgata; Dm, Drosophila melanogaster; Xl, Xenopus laevis; Hs, Homo sapiens (GIF 14 kb)
Fig. S2
Maximum likelihood consensus tree of the basic-helix-loop-helix domains of Twist family proteins from Capitella sp. I and other species. Also included are sequences of related basic-helix-loop-helix proteins, Paraxis and Hairy. Support values calculated from the maximum likelihood analysis are above the branches; values below 50 are not included. Species abbreviations are as follows: CapI, Capitella sp. I; Xl, Xenopus laevis; Ec, Enchytraeus coronatus (oligochaete) ; Am, Apis Mellifera (honey bee); Dm, Drosophila melanogaster; Io, Ilynassa obsoleta (snail); HRO, Helobdella robusta (leech); Tt, Transennella tantilla (bivalve); Dr, Danio rerio; Pv, Patella vulgata (limpet); Gg, Gallus gallus; Hs, Homo sapiens; Mm, Mus musculus; Tc, Tribolium castaneum (beetle); Nv, Nematostella vectensis (sea anemone); Pc, Podocoryne carnea, (jellyfish) (GIF 16 kb)
Fig. S3
Bayesian inference consensus tree of Snail and Slug family members from Capitella sp. I and other representative species. Sequences of Kruppel, a related zinc-finger protein, are included as an outgroup. Posterior probabilities from Bayesian analysis are reported above the branches and support values calculated from Maximum Likelihood analysis are below the branches; values below 50 are not included. Capitella sp. I genes are indicated with shaded boxes. Among the Snail family, lophotrochozoan genes are in purple and vertebrate genes are in green; the Scratch family is in blue. The two Capitella sp. I scratch genes included in the orthology analyses were isolated by degenerate PCR and RACE during our initial attempts at isolating snail fragments (Thamm and Seaver, unpublished). Species abbreviations are as follows: Hs, Homo sapiens; Mm, Mus musculus; Dr, Danio rerio; Gg, Gallus gallus; Xl, Xenopus laevis; CapI, Capitella sp. I; Pv, Patella vulgata (limpet); Nv, Nematostella vectensis (sea anemone); Am, Acropora millepora (coral); Pc, Podocoryne carnea, (jellyfish); HRO, Helobdella robusta (leech); Bf, Branchiostoma floridae (amphioxus); At, Achaearanea tepidariorum (spider); Dm, Drosophila melanogaster; Tc, Tribolium castaneum (beetle); Lf, Lithobius forficatus (centipede); Io, Ilynassa obsoleta (snail) (GIF 15 kb)
Rights and permissions
About this article
Cite this article
Dill, K.K., Thamm, K. & Seaver, E.C. Characterization of twist and snail gene expression during mesoderm and nervous system development in the polychaete annelid Capitella sp. I. Dev Genes Evol 217, 435–447 (2007). https://doi.org/10.1007/s00427-007-0153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-007-0153-4