Abstract
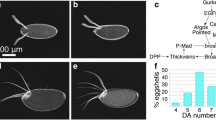
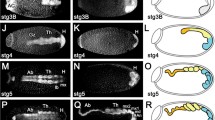
Oenocytes are a specialized cell type required for lipid processing, pheromone secretion, and developmental signaling. Their development has been well characterized in Drosophila melanogaster, but it remains unknown whether the developmental program is conserved in other insect species. In this study, we compare and contrast the specification and development of larval oenocytes between Drosophila and the red flour beetle, Tribolium castaneum. First, we identify several useful reagents to label larval oenocytes, including both a Tribolium GFP enhancer trap line and a simple flurophore-conjugated streptavidin staining method that recognizes oenocytes across insect species. Second, we use these tools to describe oenocyte development in Tribolium embryos, and our findings provide evidence for conserved roles of MAP kinase signaling as well as the Spalt, Engrailed, hepatocyte nuclear factor-4, and ventral veins lacking factors in producing abdominal-specific oenocyte cells. However, Tribolium embryos produce four times as many oenocytes per abdominal segment as Drosophila, and unlike in Drosophila, these cells rapidly downregulate the expression of the Spalt transcription factor. Thus, these results provide new insight into the molecular pathways regulating oenocyte specification across insect species.







Similar content being viewed by others
References
Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD (2009) Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461:987–991
Brodu V, Elstob PR, Gould AP (2002) Abdominal A specifies one cell type in Drosophila by regulating one principal target gene. Development 129:2957–2963
Brodu V, Elstob PR, Gould AP (2004) EGF receptor signaling regulates pulses of cell delamination from the Drosophila ectoderm. Dev Cell 7:885–895
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Eastham LES (1929) The post-embryonic development of Phaenoserphus viator Hal. (Proctotrypoidea), a parasite of the larva of Pterostichus niger (Carabidae), with notes on the anatomy of the larva. Parasitology 21:1–21
Elstob PR, Brodu V, Gould AP (2001) Spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development 128:723–732
Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD, Kravitz EA (2010) Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol 8:e1000541
Fletcher K, Myant NB (1960) Biotin in the synthesis of fatty acid and cholesterol by mammalian liver. Nature 188:585
Gabay L, Seger R, Shilo BZ (1997) In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277:1103–1106
Gebelein B, Mann RS (2007) Compartmental modulation of abdominal Hox expression by engrailed and sloppy-paired patterns the fly ectoderm. Dev Biol 308:593–605
Gould AP, Elstob PR, Brodu V (2001) Insect oenocytes: a model system for studying cell-fate specification by Hox genes. J Anat 199:25–33
Gutierrez E, Wiggins D, Fielding B, Gould AP (2007) Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445:275–280
Gutzwiller LM, Witt LM, Gresser AL, Burns KA, Cook TA, Gebelein B (2010) Proneural and abdominal Hox inputs synergize to promote sensory organ formation in the Drosophila abdomen. Dev Biol 348:231–243
Inbal A, Levanon D, Salzberg A (2003) Multiple roles for u-turn/ventral veinless in the development of Drosophila PNS. Development 130:2467–2478
Jackson A, Locke M (1989) The formation of plasma membrane reticular systems in the oenocytes of an insect. Tissue Cell 21:463–473
Kornberg T (1981) Engrailed: a gene controlling compartment and segment formation in Drosophila. Proc Natl Acad Sci U S A 78:1095–1099
Lage P, Jan YN, Jarman AP (1997) Requirement for EGF receptor signalling in neural recruitment during formation of Drosophila chordotonal sense organ clusters. Curr Biol: CB 7:166–175
Levine M (2010) Transcriptional enhancers in animal development and evolution. Curr Biol: CB 20:R754–R763
Li-Kroeger D, Witt LM, Grimes HL, Cook TA, Gebelein B (2008) Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev Cell 15:298–308
Mahadevan LC, Willis AC, Barratt MJ (1991) Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell 65:775–783
Palanker L, Tennessen JM, Lam G, Thummel CS (2009) Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab 9:228–239
Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJ et al (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452:949–955
Roth S, Hartenstein V (2008) Development of Tribolium castaneum. Dev Genes Evol 218:115–118
Rusten TE, Cantera R, Urban J, Technau G, Kafatos FC, Barrio R (2001) Spalt modifies EGFR-mediated induction of chordotonal precursors in the embryonic PNS of Drosophila promoting the development of oenocytes. Development 128:711–722
Schroder R, Beermann A, Wittkopp N, Lutz R (2008) From development to biodiversity—Tribolium castaneum, an insect model organism for short germband development. Dev Genes Evol 218:119–126
Shilo BZ (2005) Regulating the dynamics of EGF receptor signaling in space and time. Development 132:4017–4027
Shippy TD, Brown SJ, Denell RE (1998) Molecular characterization of the Tribolium abdominal-A ortholog and implications for the products of the Drosophila gene. Dev Genes Evol 207:446–452
Snodgrass RE (1993) Principles of insect morphology. Cornell University Press, Ithaca
Tomoyasu Y, Wheeler SR, Denell RE (2005) Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433:643–647
Trauner J, Schinko J, Lorenzen MD, Shippy TD, Wimmer EA, Beeman RW, Klingler M, Bucher G, Brown SJ (2009) Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol 7:73
Wheeler WM (1892) Concerning the blood tissue of insects. Psyche 6:216–258
Witt LM, Gutzwiller LM, Gresser AL, Li-Kroeger D, Cook TA, Gebelein B (2010) Atonal, senseless, and abdominal-A regulate rhomboid enhancer activity in abdominal sensory organ precursors. Dev Biol 344:1060–1070
Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T (2007) Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development 134:4243–4253
Zara FJ, Caetano FH (2004) Ultramorphology and histochemistry of fat body cells from last instar larval of the Pachycondyla (=Neoponera) villosa (Fabricius) (Formicidae: Ponerinae). Braz J Biol 64:725–735
Ziegler R, Engler DL, Davis NT (1995) Biotin-containing proteins of the insect nervous system, a potential source of interference with immunocytochemical localization procedures. Insect Biochem Mol Biol 25:569–574
Acknowledgments
We thank Alex Gould, GEKU, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank (University of Iowa) for the reagents. We thank Tingjia Lao and Padmapriyadarshini Ravisankar for technical assistance. This work was supported by an NIH grant GM079428A to B.G and an NSF grant (IOS0950964) to Y.T.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1,925 kb)
Rights and permissions
About this article
Cite this article
Burns, K.A., Gutzwiller, L.M., Tomoyasu, Y. et al. Oenocyte development in the red flour beetle Tribolium castaneum . Dev Genes Evol 222, 77–88 (2012). https://doi.org/10.1007/s00427-012-0390-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-012-0390-z