Abstract
According to observations in various cell lines, elimination of the cyclin-dependent kinase inhibitor p27(KIP1) during the late G1 phase of the cell cycle is required for progression to the S phase. Eyes from C57BL/6 mice at embryonic days 13, 14, and 18, and at 4 weeks of age, were analyzed by a bromodeoxyuridine cell proliferation assay and by immunocytochemistry using anti-p27(KIP1) antibody. On embryonic days 14 and 18, p27(KIP1) was detected in the ciliary body. This protein also was detected in the nuclei of the many cells of the retinal pigment epithelium on embryonic day 18, and was present in all such cells at 4 weeks of age. When p27(KIP1)-/- knockout and control mice were injected with bromodeoxyuridine between postnatal days 7 and 10 and analyzed on day 11, positive cells were abundant in the retinal pigment epithelium and the ciliary body of p27(KIP1)-/- mice, whereas few cells were positive in control mice. By fluorescent nuclear staining in whole mounts of retinal pigment epithelium at 12 weeks of age, more nuclei were present in p27(KIP1)-/- than in the wild-type mice. These results suggest that p27(KIP1) was involved in regulation of proliferation in the RPE and the ciliary body.
Similar content being viewed by others
Introduction
Early in ocular development, the thick inner layer of the optic cup consists of elongated neuroepithelial cells destined to make up the future visual retina and the ciliary body retina. Later the visual retina shows increased cell division and becomes thicker, whereas the ciliary body retina shows a decrease in dividing cells and becomes thinner in comparison with the visual retina (Kuwabara and Weidman 1974; Bard et al. 1982); however, the mechanisms regulating cell proliferation in the optic cup remain to be determined.
Cell cycle progression is controlled by a series of kinase complexes composed of cyclins and cyclin-dependent kinases (CDKs; Sherr and Roberts 1995). Enzymatic activities of cyclin/CDK complexes are regulated by many mechanisms that reflect both the diversity of the signals that they integrate and the central importance of their roles in cell cycle control. These regulatory mechanisms include the actions of CDK inhibitors (CKIs; Toyoshima and Hunter 1994; Kato et al. 1994). One of such CKI is p27(KIP1). In various cell lines, elimination of p27(KIP1) during the late G1 phase has been shown to be required for cell cycle progression to the S phase (Nourse et al. 1994; Reynisdottir et al. 1995; Coats et al. 1996; Hirai et al. 1997).
Previous studies indicated that p27(KIP1) mRNA does not fluctuate during the cell cycle, implying that control of p27(KIP1) expression may be posttranslational (Polyak et al. 1994). The ubiquitin-mediated proteosome pathway was suggested to be involved in p27(KIP1) degradation in mammals (Pagano et al. 1995). This proteosome pathway is emerging as a major universal mechanism that regulates selective and time-controlled elimination of key short-lived regulatory proteins such as CKIs (Blagosklonny et al. 1996) and IkB (Thanos et al. 1995). The pathway has been suggested to be involved in differentiation of corneal epithelial cells (Yoshida et al. 2000). We have recently reported that degradation of p27(KIP1) is involved in regulation of proliferation in response to wounding of the corneal epithelium (Yoshida et al. 2002a). In the present study, the expression of p27(KIP1) was examined during the development of the mouse eye. In addition, proliferation of the developing ciliary body and the retinal pigment epithelium (RPE) was compared between p27(KIP1)-/- knockout mice and control mice.
Materials and methods
Development of p27(KIP1)-/- mice
Cloned genomic DNA corresponding to the p27(KIP1) locus was isolated from a 129/Sv mouse genomic library (Nakayama et al. 1996). To produce the p27(KIP1)-/- mice, a targeting vector was constructed by replacing a 2.5-kb SmaI-SmaI fragment containing the entire p27(KIP1) sequence with a PGK-neo-poly(A) cassette. Maintenance, transfection, and selection of ES cells were performed as described previously (Nakayama et al. 1996). Mutant ES cells were microinjected into C57BL/6, and the resulting male chimeras were mated with female C57BL/6 mice.
Animals and tissues
C57BL/6 mouse embryos of embryonic day (E)13, E14, and E18 stages were removed by dissection from the pregnant mice anesthetized with a ketamine cocktail (ketamine 3.75 ml at 100 mg/ml, acepromazine 0.3 ml at 10 mg/ml, rompun/xylazine 1.9 ml at 20 mg/ml, and sterile saline 23 ml) at dose of 0.4 ml/100 g body weight. For bromodeoxyuridine (BrdU) labeling, the pregnant mouse was injected intraperitoneally with BrdU at a dose of 30 mg/kg body weight at 30 min before removal of embryos. Eyes also were dissected from C57BL/6 mice 4 weeks after birth. The p27(KIP1) knockout mice and littermate controls were injected with BrdU (30 mg/kg) once on P7, twice per day on P8 and P 9, and once on P10 and killed on P11. Embryos or dissected eyes were washed in saline and fixed in ice-cold 4% paraformaldehyde (PFA) in 0.1 M borate buffer (pH 9.5) for 2 h, and processed for paraffin sectioning. The animal experiments conformed to the ARVO Resolution on the Use of Animals in Research.
Immunocytochemistry
Sections were dewaxed and rehydrated and then rinsed twice in phosphate-buffered saline (PBS) and incubated with normal goat serum and then with a 1:1000 dilution of p27(KIP1) antibody (BD Bioscience Pharmingen, San Diego, Calif.), which was generated using mouse p27(KIP1). Binding of the primary antibody was localized by fluorescence microscopy using FITC conjugated goat anti-mouse IgG (Jackson Immuno Research Laboratories, West Grove, Pa.) at a dilution of 1:200. Anti-BrdU staining was performed as described previously (Yoshida et al. 2002b). BrdU-positive cells in the nonpigmented layer in the ciliary body within 200 mm from the retina were counted in six eyes from wild-type and six from p27(KIP1)-/-mice. Significance was analyzed by Aspin-Welch’s t test.
Whole mount of RPE
The p27(KIP1) knockout and wild-type mice (12 weeks of age) were anesthetized with ketamine cocktail and perfused transcardially with 5 ml of saline followed by 20 ml of 4% PFA in 0.1 M PBS. The eyes were dissected, and a small tab of lid tissue was retained at the 12 o’clock position for orientation. Specimens were kept in 4% PFA in PBS for 24 h and in PBS for another 24 h. The lens then was removed, and the posterior eye cup was laid flat on a glass slice after making a set of eight radial incisions through the ora serrata region (Bodenstein et al. 1987). The tab of lid tissue was cut away and, to retain orientation, a notch was cut into the dorsalmost “petal” at the 12 o’clock position. The retina then was removed, and remaining posterior tissues were mounted flat on a gelatinized glass slide with the RPE facing upward. This whole mount was air dried for 1 h, stained with Hoechst dye, and finally rinsed with water before examination with an Axiovert 35-M microscope equipped for epifluorescence (Zeiss).
Statistical analysis
The whole mount was divided into four quadrants. In each quadrant, a microscopic field of identical size (0.0817 mm2) was chosen at approximately the same distance (0.75 mm) from the optic disc to count nuclei in the RPE (Yoshida et al. 2002a). Seven wild-type mice and seven p27(KIP1)-/-mice were used. Results were evaluated using Aspin-Welch’s t test.
Results
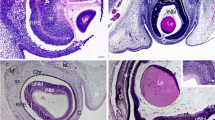
At E13, the thick inner layer of the optic cup consists of elongated neuroepithelial cells that will become the visual retina and ciliary body retina (Fig. 1b). At this stage, BrdU-positive cells were detected among the epithelial cells of the lens (Fig. 1c) and p27(KIP1) was expressed in primary lens fiber cells (Fig. 1a).
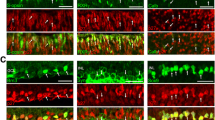
Immunohistochemical analyses in embryonic and postnatal mouse eyes. a–i The immunodetection of p27(KIP1) (a,d,g) and bromodeoxyuridine (BrdU; c,f,i), and as well as hematoxylin and eosin (H&E) staining for anatomic reference (b,e,h) in the C57Bl/6 mouse eye on embryonic day (E) 13 (a–c), E14 (d–f), and E18 (g–i) On E13, p27(KIP1) was expressed by primary lens fiber cells (a, arrowhead) On E14, p27(KIP1) was expressed in both the ciliary body retina (d, arrow) and the inner portion of the visual retina (d, arrowhead) On E18, p27(KIP1) was expressed in many cells of the ciliary body retina (g, arrowhead) and in a few cells of the visual retina. At this stage, few cells in the ciliary body retina were positive for BrdU (f) j–l Immunodetection of p27(KIP1) (j) and glutamate synthetase (k), as well as H&E staining (l) in the 4-week-old C57BL/6 mouse retina. The p27(KIP1)-positive nuclei were distributed in the middle sublayer of the inner nuclear layer (INL; j), where glutamate synthetase positive cells also were seen (k). m,n Respectively show immunodetection of p27(KIP1) and the nuclear staining with Hoechst dye in the retinal pigment epithelium (RPE) in the same section. All nuclei in the RPE were positive for p27(KIP1) Scale bar=200 mm for panels a–c, 170 mm for d–i), 100 mm for j–l, and 40 mm for m and n
On E14 and E18, the visual retina showed cell division as well as overall thickening, whereas the ciliary body retina had few dividing cells and became more thin as compared with the visual retina (Fig. 1e,f,h,i). Both the ciliary body retina (Fig. 1d, arrow, g, arrowhead) and the inner region of the visual retina showed p27(KIP1) expression (Fig. 1d, arrowhead). On E18, many RPE cells were immunoreactive for p27(KIP1) (Fig. 1 g).
In 4-week-old mice, p27(KIP1) was expressed in the middle sublayer of the inner nuclear layer (INL) of the retina and RPE (Fig. 1j,m). Additional immunostaining of the glutamate synthetase (Fig. 1k), a marker for Müller cells (Harada et al. 1998), suggested that Müller cells express p27(KIP1). All RPE cells were positive for p27(KIP1) as indicated by nuclear staining in the same section (Fig. 1n).
To examine the role of p27(KIP1) in development of the ciliary body and RPE, the p27(KIP1) knockout mice and littermate controls were injected with BrdU (30 mg/kg) once on P7, twice per day on P8 and P9, and once on P10 and analyzed on P11. BrdU-positive cells were not detected in the central retina of wild-type mice (Fig. 2b) but they were detected in the inner and outer nuclear layer (INL and ONL), and the RPE of the p27(KIP1)-/- mouse (Fig. 2d). Ciliary bodies from p27(KIP1)-/- mice (Fig. 2h,l,p) contained more BrdU-positive cells than those from wild-type mice (Fig. 2f, j,n). The number of BrdU-positive cells in the nonpigmented layer of the ciliary body within 200 mm of the retina was 27.9±13.6 (mean±standard deviation) in p27(KIP1)-/- mice, significantly different from findings in wild-type mice (4.3±1.5), according to Aspin-Welch’s t test (P=0.00393).
Nissl staining (a,c,e,g,i,k,m,o) and bromodeoxyuridine (BrdU) staining (b,d,f,h,j,l,n,p) in the retina (a–d) and the ciliary body (e–p) of wild-type (a,b,e,f,i,j,m,n) and p27(KIP1)-/- (c,d,g,h,k,l,o,p) mice. Mice were injected with BrdU (30 mg/kg) once on P7, twice per day on P8 and P 9, and once on P10 and analyzed on P11. BrdU-positive cells were not detected in the central retina of wild-type mice, but they were demonstrated in the inner and outer nuclear layers, and retinal pigment epithelium of the central retina of p27(KIP1)-/- mice. The ciliary body contained more BrdU-positive cells in p27(KIP1)-/- mice than in wild-type mice. The ciliary bodies shown in e,i,m were from different animals. Scale bar=80.5 mm for a–d and 200 mm for panels e–p
Finally, nuclear staining with Hoechst dye was performed the whole mounts of the RPE. Figure 3 shows representative view of RPE of the 12-week-old wild-type and p27(KIP1)-/- mice. More nuclei were seen in the RPE of p27(KIP1)-/- mice than the wild-type mice. The ratio of stained RPE cell nuclei (mean±standard deviation) in p27(KIP1)-/- mice to that in wild-type mice was 2.13±0.29 (P=0.00000562).
Discussion
In this study, p27(KIP1) was expressed in the ciliary body on E14 and E18, a time when the ciliary body retina was terminating division and becoming thinner in comparison with the visual retina, whereas p27(KIP1) was detected in the RPE on E18 and also 4 weeks after birth. Mechanisms regulating expression of p27(KIP1) during development of the eye remain to be determined. Previous studies showed that p27(KIP1) mRNA does not fluctuate during the cell cycle, implying the existence of posttranslational processes controlling the degree of p27(KIP1) expression (Polyak et al. 1994). The ubiquitin-mediated proteosome pathway has been suspected to be involved in p27(KIP1) degradation in mammals (Pagano et al. 1995). We have reported that p27(KIP1) was also eliminated by ubiquitin-independent processing (Shirane et al. 1999). Identification of mechanisms regulating p27(KIP1) expression should prove valuable in delineating molecular pathways underlying development of the eye.
The p27(KIP1) protein prevents a cell in the G phase of the cell cycle from entering the S phase (Kato et al. 1994; Polyak et al. 1994; Toyoshima et al. 1994; Harper and Elledge 1996). When the p27(KIP1) knockout mice and littermate controls were injected with BrdU (30 mg/kg) once on P7, twice per day on P8 and P9, and once on P10 and analyzed on P11, BrdU-positive cells were detected in the RPE and the ciliary body of the p27(KIP1)-/- mice, but few RPE or ciliary body cells were positive in control mice. These results suggest that in the ciliary body and the RPE of wild-type mice, proliferation ceased up to P7, whereas in p27(KIP1)-/- mice proliferation persisted after P7, as has been reported previously in the retina (Levine et al. 2000; Dyer and Cepko 2000).
As for nuclear staining in the RPE whole mount of at 12 weeks of age, the ratio of the number of stained nuclei in RPE cells of p27(KIP1)-/- mice to stained RPE cell numbers in wild-type mice was 2.13. This result disagreed with the previous reports arguing against a dramatic increase in absolute cell numbers of corneal epithelial cells in p27(KIP1)-deficient mice (Yoshida et al. 2002b). Involvement of p27(KIP1)-related protein in cell proliferation may have differed by species and by tissue. The related CDK-inhibitor proteins in Caenorhabditis elegans and Drosophila, cki-1 and dacapo, respectively, are expressed similarly to p27(KIP1) during embryogenesis in cells from several lineages (de Nooij et al. 1996; Hong et al. 1998; Lane et al. 1996). In Drosophila, dacapo mutants failed to show arrest in epidermal cells at the appropriate time, leading to one or two extra rounds of division (de Nooij et al. 1996; Lane et al. 1996). Similarly, inactivation of cki-1 by RNA-interference in C. elegans produced ectopic cell division in several lineages (Hong et al. 1998). Other CDK-inhibitor proteins could compensate for the lack of p27(KIP1), as has been reported in lens, lung, and muscle development (Zhang et al. 1998; Zhang et al. 1999). In fact, it is impossible to count the number of cells in the ciliary body of the adult mice. But we think total number of cells in the nonpigmented layer of the ciliary body of the adult mice was more than wild-type mice from the BrdU data.
Differences in absolute cell number between wild-type and p27(KIP1)-/- mice may have resulted from differential apoptosis; however, tunel assay with at P2 and P11 of both p27(KIP1) knockout mice and littermate controls did not demonstrate any positive cells in the RPE (data not shown). Apparently, differences in the number of nuclei in the RPE were not caused by changes in cell death.
The additionally produced cells in the RPE (Nakayama et al. 1996) and ciliary body (data not shown) were fully differentiated in the 12-week-old knockout animals by H&E staining, suggesting that p27(KIP1) did not play an important role in differentiation of ciliary body and RPE.
References
Bard JB, Ross AS (1982) The morphogenesis of the ciliary body of the avian eye. I. Lateral cell detachment facilitates epithelial folding. Dev Biol 92: 73–86
Blagosklonny MV, Wu GS, Omura S, el-Deiry WS (1996) Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem Biophys Res Commun 227:564–569
Bodenstein L, Sidman RL (1987) Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev Biol 121:192–204
Coats S, Flanagan WM, Nourse J, Roberts JM (1996) Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272:877–880
de Nooij JC, Letendre MA, Hariharan IK (1996) A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell 87:1237–1247
Dyer MA, Cepko CL (2000) Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci 3:873–880
Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, Sasaki S, Okuyama S, Watase K, Wada K, Tanaka K (1998) Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci USA 95:4663–4666
Harper JW, Elledge SJ (1996) Cdk inhibitors in development and cancer. Curr Opin Genet Dev 6:56–64
Hirai A, Nakamura S, Noguchi Y, Yasuda T, Kitagawa M, Tatsuno I, Oeda T, Tahara K, Terano T, Narumiya S, Kohn LD, Saito Y (1997) Geranylgeranylated rho small GTPase(s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. J Biol Chem 272:13–16
Hong Y, Roy R, Ambros V (1998) Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125:3585–3597
Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ (1994) Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79:487–496
Kuwabara T, Weidman TA (1974) Development of the prenatal rat retina. Invest Ophthalmol 13:725–739
Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87:1225–1235
Levine EM, Close J, Fero M, Ostrovsky A, Reh TA (2000) p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev Biol 219:299–314
Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY (1996) Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707–720
Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM (1994) Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570–573
Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M (1995) Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682–685
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59–66
Reynisdottir I, Polyak K, Lavarone A, Massague J (1995) Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 9:1831–1845
Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9:1149–1163
Shirane M, Harumiya Y, Ishida N, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M (1999) Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem 274:13886–13893
Thanos D, Maniatis T (1995) NF-kappa B: a lesson in family values. Cell 80:529–532
Toyoshima H, Hunter T (1994) p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78:67–74
Yoshida K, Hu Y, Karin M (2000) IkappaB Kinase alpha Is essential for development of the mammalian cornea and conjunctiva. Invest Ophthalmol Vis Sci 41:3665–3669
Yoshida K, Behrens A, Le-Niculescu H, Wagner EF, Harada T, Imaki J, Ohno S, Karin M (2002a) Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve Ttransection. Invest Ophthalmol Vis Sci 43:1631–1635
Yoshida K, Nakayama K, Nagahama H, Harada T, Harada C, Imaki J, Matsuda A, Yamamoto K, Ito M, Ohno S, Nakayama KI (2002b) Involvement of p27(KIP1) degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest Ophthalmol Vis Sci 43:364–370
Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ (1998) Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev 12:3162–3167
Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ (1999) p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev 13:213–224
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, K., Nakayama, K., Kase, S. et al. Involvement of p27(KIP1) in proliferation of the retinal pigment epithelium and ciliary body. Anat Embryol 208, 145–150 (2004). https://doi.org/10.1007/s00429-004-0382-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0382-5