Abstract
Neutrophil extracellular traps (NETs) have recently been discovered as a central part of antimicrobial innate immunity. In the meanwhile, evidence accumulated that NETs are also generated upon non-infectious stimuli in various clinical settings. In acute or chronic inflammatory disorders aberrantly enhanced NET formation and/or decreased NET degradation seems to correlate with disease outcome. This review summarizes current knowledge about the relation of NETs in a broad spectrum of clinical settings. Specifically, we focus on the importance of NETs as a predictive marker in severely ill patients and further, we speculate about the potential pathophysiology of NETs.


Similar content being viewed by others
References
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241
Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B (2006) An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16:401–407
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13:463–469
Medina E (2009) Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J Innate Immun 1:176–180
Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S (2006) Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann NY Acad Sci 1075:118–122
Valeva A, Walev I, Weis S, Boukhaillouk F, Wassenaar TM, Bhakdi S (2008) Pro-inflammatory feedback activation cycle evoked by attack of Vibrio cholerae cytolysin on human neutrophil granulocytes. Med Microbiol Immunol 197:285–293
Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, Garlanda C, Romani L, Gascan H, Bellocchio S, Bozza S, Cassatella MA, Jeannin P, Mantovani A (2007) The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 204:793–804
Curran CS, Demick KP, Mansfield JM (2006) Lactoferrin activates macrophages via TLR4-dependent and -independent signalling pathways. Cell Immunol 242:23–30
Zhang LT, Yao YM, Lu JQ, Yan XJ, Yu Y, Sheng ZY (2008) Recombinant bactericidal/permeability-increasing protein inhibits endotoxin-induced high mobility group box 1 protein gene expression in sepsis. Shock 29:278–284
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5:577–582
Lee WL, Grinstein S (2004) Immunology. the tangled webs that neutrophils weave. Science 303:1477–1478
Scholz M, Cinatl J (2005) Fas/FasL interaction: a novel immune therapy approach with immobilized biologicals. Med Res Rev 25:331–342
Jana S, Paliwal J (2007) Apoptosis: potential therapeutic targets for new drug discovery. Curr Med Chem 14:2369–2379
Urban CF, Reichard U, Brinkmann V, Zychlinsky A (2006) Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8:668–676
Lehrer RI, Ganz T (1999) Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol 11:23–27
Ganz T, Oren A, Lehrer RI (1992) Defensins: microbicidal and cytotoxic peptides of mammalian host defense cells. Med Microbiol Immunol 181:99–105
von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E (2008) Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111:3070–3080
Sumby P (2005) Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA 102:1679–1684
Puyet A, Greenberg B, Lacks SA (1990) Genetic and structural characterization of endA. A membrane-bound nuclease required for transformation of Streptococcus pneumoniae. J Mol Biol 213:727–738
Wassenaar TM, Engelskirchen M, Park S, Lastovica A (1997) Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med Microbiol Immunol 186:139–144
Mandell GL (1975) Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal–leukocyte interaction. J Clin Invest 55:61–66
Hong W, Juneau RA, Pang B, Swords WE (2009) Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun 1:215–224
Palić D, Ostojić J, Andreasen CB, Roth JA (2007) Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev Comp Immunol 31:805–816
Jacobs T, Andrä J, Gaworski I, Graefe S, Mellenthin K, Krömer M, Halter R, Borlak J, Clos J (2005) Complement C3 is required for the progression of cutaneous lesions and neutrophil attraction in Leishmania major infection. Med Microbiol Immunol 194:143–149
Wartha F, Beiter K, Normark S, Henriques-Normark B (2007) Neutrophil extracellular traps: casting the NET over pathogenesis. Curr Opin Microbiol 10:52–56
Schultz H (2007) From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI). Autoimmun Rev 6:223–227
McKenzie SG, Chowdhury S, Strandvik B, Hodson ME (2007) Dornase alfa is well tolerated: data from the epidemiologic registry of cystic fibrosis. Pediatr Pulmonol 42:928–937
Osterloh A, Breloer M (2008) Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol 197:1–8
Steinberg BE, Grinstein S (2007) Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE 2007:pe11
Stef G (2007) Inhibition of NAD(P)H oxidase attenuates aggregation of platelets from high-risk cardiac patients with aspirin resistance. Pharmacol Rep 59:428–436
Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V (2009) Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 1:181–193
Swarup V, Rajeswari MR (2007) Circulating (cell-free) nucleic acids—a promising, non-invasive tool for early detection of several human diseases. FEBS Lett 581:795–799
Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J (2008) Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 30:352–358
Lögters T, Paunel-Görgülü A, Zilkens C, Altrichter J, Scholz M, Thelen S, Krauspe R, Margraf S, Jeri T, Windolf J, Jäger M (2009) Diagnostic accuracy of neutrophil-derived circulating free DNA (cf-DNA/NETs) for septic arthritis. J Orthop Res. [Epub ahead of print]
Palić D, Andreasen CB, Ostojić J, Tell RM, Roth JA (2007) Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J Immunol Methods 319:87–97
Pattison DI, Davies MJ (2006) Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem 13:3271–3290
Wang Y, Rosen H, Madtes DK, Shao B, Martin TR, Heinecke JW, Fu X (2007) Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J Biol Chem 282:31826–31834
Ioachimescu OC, Stoller JK (2005) A review of alpha-1 antitrypsin deficiency. COPD 2:263–275
Klebanoff SJ, Kinsella MG, Wight TN (1993) Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase-H2O2-chloride system. Am J Pathol 143:907–917
Fujishima S, Morisaki H, Ishizaka A, Kotake Y, Miyaki M, Yoh K, Sekine K, Sasaki J, Tasaka S, Hasegawa N, Kawai Y, Takeda J, Aikawa N (2008) Neutrophil elastase and systemic inflammatory response syndrome in the initiation and development of acute lung injury among critically ill patients. Biomed Pharmacother 62:333–338
Knapp S, Hareng L, Rijneveld AW, Bresser P, van der Zee JS, Florquin S, Hartung T, van der Poll T (2004) Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J Infect Dis 189:1506–1515
Propst-Graham KL, Preheim LC, Vander Top EA, Snitily MU, Gentry-Nielsen MJ (2007) Cirrhosis-induced defects in innate pulmonary defenses against Streptococcus pneumoniae. BMC Microbiol 7:94
Sun K, Salmon SL, Lotz SA, Metzger DW (2007) Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun 75:1196–1202
Bianchi M, Hakkim Rahamathullah A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood [Epub ahead of print]
Cinatl J Jr, Michaelis M, Doerr HW (2007) The threat of avian influenza a (H5N1): part II: clues to pathogenicity and pathology. Med Microbiol Immunol 196:191–201
Desloges N, Schubert C, Wolff MH, Rahaus M (2008) Varicella-zoster virus infection induces the secretion of interleukin-8. Med Microbiol Immunol 197:277–284
Zhou J, Yang XQ, Fu Z, Zhao XD, Jiang LP, Wang LJ, Cui YX (2008) Increased pathogenesis and inflammation of airways from respiratory syncytial virus infection in T cell deficient nude mice. Med Microbiol Immunol 197:345–351
von Müller L, Mertens T (2008) Human cytomegalovirus infection and antiviral immunity in septic patients without canonical immunosuppression. Med Microbiol Immunol 197:75–82
Robinson P (2001) Cystic fibrosis. Thorax 56:237–241
Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME (1994) Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331:637–642
Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, Henriques-Normark B (2007) Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 9:1162–1171
Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, Simpson AJ, Buchanan JT, Chhatwal GS, Kotb M, Nizet V (2007) DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med 13:981–985
Mrabat H, Beagle J, Hang Z, Garg HG, Hales CA, Quinn DA (2009) Inhibition of HA synthase 3 mRNA expression, with a phosphodiesterase 3 inhibitor, blocks lung injury in a septic ventilated rat model. Lung [Epub ahead of print]
Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA (2008) High-molecular-weight hyaluronan—a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12:R102
Kitz R, Rose MA, Placzek K, Schulze J, Zielen S, Schubert R (2008) LPS inhalation challenge: a new tool to characterize the inflammatory response in humans. Med Microbiol Immunol 197:13–19
Shin HD, Park BL, Kim LH, Lee HS, Kim TY, Bae SC (2004) Common DNase I polymorphism associated with autoantibody production among systemic lupus erythematosus patients. Hum Mol Genet 13:2343–2350
Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stüber F (2008) A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol 197:313–324
Ma AC, Kubes P (2007) Platelets, neutrophils, and extracellular traps (NETs) in sepsis. J Thromb Haemost 6:415–420
Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15:623–625
Kamgang RK, Ramos I, Rodrigues Duarte L, Ghielmetti M, Freudenberg M, Dahinden C, Padovan E (2008) Using distinct molecular signatures of human monocytes and dendritic cells to predict adjuvant activity and pyrogenicity of TLR agonists. Med Microbiol Immunol 197:369–379
Hsieh SC, Yu HS, Cheng SH, Li KJ, Lu MC, Wu CH, Tsai CY, Yu CL (2007) Anti-myeloperoxidase antibodies enhance phagocytosis, IL-8 production, and glucose uptake of polymorphonuclear neutrophils rather than anti-proteinase 3 antibodies leading to activation-induced cell death of the neutrophils. Clin Rheumatol 26:216–224
Manderson AP, Carlucci F, Lachmann PJ, Lazarus RA, Festenstein RJ, Cook HT, Walport MJ, Botto M (2006) The in vivo expression of actin/salt-resistant hyperactive DNase I inhibits the development of anti-ssDNA and anti-histone autoantibodies in a murine model of systemic lupus erythematosus. Arthritis Res Ther 8:R68
Parseghian MH, Luhrs KA (2006) Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity. Biochem Cell Biol 84:589–604
Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, Sagay SA, Egah DZ, Iya D, Afolabi BB, Baker M, Ford K, Ford R, Roux KH, Keller TCIII (2008) Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J 7:41
Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906–917
Acknowledgments
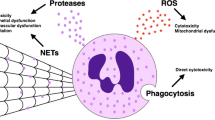
We thank Julia Quathamer for the fluorescence staining and photographs of NETs and Coelestina Scholz for the graphics depicted in Fig. 2. We appreciated the critical review of the manuscript by Dr Vidya Prasad and the support of Rose Garden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lögters, T., Margraf, S., Altrichter, J. et al. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol 198, 211–219 (2009). https://doi.org/10.1007/s00430-009-0121-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-009-0121-x