Abstract
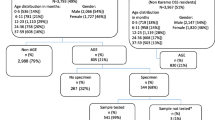
This observational, prospective study was undertaken to estimate the burden of rotavirus (RV) gastroenteritis (GE) leading to general practitioner (GP)/family paediatrician (FP) visits among children aged <5 years in Czech Republic, Germany, Italy, Poland, Spain and the UK. Children aged <5 years presenting with acute GE provided stool samples for rapid RV testing. RV+ samples were confirmed and typed by RT-PCR. Demographic and clinical data were collected for all RVGE episodes. Transmission patterns among other household children aged <5 years were also assessed. From November 2005 to May 2007, excluding data from the UK, 497/3,813 (13.0%) children aged <5 years presenting with acute GE to GP/FP and tested were RV+ by PCR. Most RVGE cases (69.1%) occurred in children aged <2 years, occurred between December and May (93.1%) and were moderate or severe by Vesikari score (92.9%). RV strain distribution varied between countries: G9P[8] was the most common type in Poland (54/76) and Spain (172/196), G1P[8] was predominant in the Czech Republic (56/64) and Italy (46/107), and G4P[8] and G1P[8] both prevailed in Germany (17/54 and 13/54, respectively). A total of 24/122 (19.7%) children aged <5 years resident in the same household as a PCR+ study participant also developed RVGE. Conclusion. This multinational epidemiological study in Europe shows that RV is easily transmitted among household children, with RVGE burden highest among children aged <2 years accessing primary healthcare for acute GE.




Similar content being viewed by others
Abbreviations
- RV:
-
Rotavirus
- GE:
-
Gastroenteritis
- GP:
-
General practitioner
- FP:
-
Family paediatrician
- PCR:
-
Polymerase chain reaction
References
Ansaldi F, Lai P, Valle L, Paediatric Leghorn Group et al (2008) Burden of rotavirus-associated and non-rotavirus-associated diarrhea among nonhospitalized individuals in central Italy: a 1-year sentinel-based epidemiological and virological surveillance. Clin Infect Dis 46:e51–e55
Antunes H, Afonso A, Iturriza M et al (2009) G2P[4] the most prevalent rotavirus genotype in 2007 winter season in an European non-vaccinated population. J Clin Virol 45:76–78
Centers for Disease Control and Prevention (CDC) (2009) Reduction in rotavirus after vaccine introduction—United States, 2000–2009. MMWR Morb Mortal Wkly Rep 58:1146–1149
Chang HG, Smith PF, Tserenpuntsag B et al (2010) Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine 28:754–758
Clark HF, Lawley D, Mallette LA et al (2009) Decline in cases of rotavirus gastroenteritis presenting to The Children's Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol 16:382–386
Coris BioConcept. RotaStrip. Rapid diagnostic test for in vitro detection of rotavirus in stool specimens. Available at: http://www.corisbio.com/public/product/documents/Rota.pdf. Accessed August 2010
Dennehy PH (2000) Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J 19:S103–S105
Desselberger U, Wolleswinkel-van den Bosch J, Mrukowicz J et al (2006) Rotavirus types in Europe and their significance for vaccination. Pediatr Infect Dis J 25(1 Suppl):S30–S41
Díez-Domingo J, Martín IO, Sanz AB et al (2006) Rotavirus gastroenteritis among children under five years of age in Valencia, Spain. Pediatr Infect Dis J 25:455–457
D'Souza RM, Hall G, Becker NG (2008) Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect 136:56–64
Forster J, Guarino A, Parez N, Rotavirus Study Group et al (2009) Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children aged <5 years. Pediatrics 123:e393–e400
Freedman SB, Eltorky M, Gorelick M, Pediatric Emergency Research Canada Gastroenteritis Study Group (2010) Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics 125:e1278–e1285
Gentsch JR, Hull JJ, Teel EN, collaborating laboratories of the National Rotavirus Strain Surveillance System et al (2009) G and P types of circulating rotavirus strains in the United States during 1996–2005: nine years of prevaccine data. J Infect Dis 200(Suppl 1):S99–S105
Grimprel E, Garbarg-Chenon A, Pirçon J-Y et al (2010) Surveillance to estimate the burden of rotavirus gastroenteritis in children aged less than 3 years attending day care centers in Paris, France. Hum Vaccines 6 (in press)
Health Protection Agency. Respiratory Syncytial Virus (RSV). Available at: http://www.hpa.org.uk/HPA/Topics/InfectiousDiseases/InfectionsAZ/1191942172184/. Accessed August 2010
Health Protection Agency. Seasonal Influenza. Available at: http://www.hpa.org.uk/HPA/Topics/InfectiousDiseases/InfectionsAZ/1191942171468/. Accessed August 2010
Iturriza-Gómara M, Kang G, Gray J (2004) Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol 31:259–265
Iturriza-Gómara M, Dallman T, Bányai K et al (2009) Rotavirus surveillance in Europe, 2005–2008: web-enabled reporting and real-time analysis of genotyping and epidemiological data. J Infect Dis 200(Suppl 1):S215–S221
Koopmans M, Brown D (1999) Seasonality and diversity of Group A rotaviruses in Europe. Acta Paediatr Suppl 88:14–19
Kühne Simmonds M, Armah G, Asmah R et al (2008) New oligonucleotide primers for P typing of rotavirus strains: strategies for typing previously untypable strains. J Clin Virol 42:368–373
Lambert SB, Faux CE, Hall L et al (2009) Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 191:157–160
National Institute for Health and Clinical Excellence. Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. April 2009. Available at: http://www.nice.org.uk/nicemedia/pdf/CG84FullGuideline.pdf. Accessed August 2010
NHS Choices. Diarrhoea. Available at: http://www.nhs.uk/conditions/diarrhoea/Pages/Introduction.aspx?url=Pages/What-is-it.aspx. Accessed August 2010
Parashar UD, Burton A, Lanata C et al (2009) Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 200(Suppl 1):S9–S15
Parashar UD, Gibson CJ, Bresse JS, Glass RI (2006) Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12:304–330
Parashar UD, Hummelman EG, Bresee JS et al (2003) Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 9:565–572
Paulke-Korinek M, Rendi-Wagner P, Kundi M et al (2010) Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in Austrian children. Pediatr Infect Dis J 29:319–323
Pickering LK, Bartlett AV 3rd, Reves RR, Morrow A (1988) Asymptomatic excretion of rotavirus before and after rotavirus diarrhea in children in day care centers. J Pediatr 112:361–365
Richardson S, Grimwood K, Gorrell R et al (1998) Extended excretion of rotavirus after severe diarrhoea in young children. Lancet 351:1844–1848
Ruuska T, Vesikari T (1990) Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 22:259–267
Santos N, Hoshino Y (2005) Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 15:29–56
Van Damme P, Giaquinto C, Huet F, on behalf of the REVEAL Study Group et al (2007) Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL Study. J Infect Dis 195:S4–S16
Van Damme P, Giaquinto C, Maxwell M, on behalf of the REVEAL Study Group et al (2007) Distribution of rotavirus genotypes in Europe, 2004–2005: the REVEAL Study. J Infect Dis 195:S17–S25
Van Effelterre T, Soriano-Gabarró M, Debrus S et al (2010) A mathematical model of the indirect effects of rotavirus vaccination. Epidemiol Infect 138:884–897
World Health Organization (2007) Rotavirus vaccines. WHO position paper. Wkly Epidemiol Rec 82:285–296
World Health Organization (2009) Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations. Wkly Epidemiol Rec 84:220–326
Acknowledgements
This study was funded by GlaxoSmithKline Biologicals. The study sponsor designed the study in collaboration with investigators, and coordinated data gathering, analysis and interpretation, and writing of the report. Authors had full access to all study data and final responsibility for the decision to submit for publication.
We would like to thank the following persons for their contribution to the study:
Manuel Martínez-Pons (Spain), Jose Villaroya (Spain), Luis Blesa (Spain), Inmaculada Latorre (Spain), Pilar Albors-Esteve (Spain), Anton Crespo (Spain), Maria Dolores Gallego (Spain), Maria Mercedes Garcia-Ballesta (Spain), Maria Desamparados Gil-Mary (Spain), Marta Graullera-Millas (Spain), Teresa Margarit-Vercher (Spain), Maria Jesus Muñoz (Spain), Amelia Peris-Vidal (Spain), Pedro Polo-Martin (Spain), Alesandra Puertes-Almenar (Spain), Maria José Sanz (Spain), Ignacio Sorribes-Monrabal (Spain), Pilar García-Corbeira (GSK Spain), Eduardo Fernández (GSK Spain), Sandra Sistiaga (GSK Spain), Saúl Robles (GSK Spain), Silvia Della Coletta (GSK Italy), Federico Marchetti (GSK Italy), Alicja Ksiazek (GSK Poland), Jana Forejtova (Czech Republic), Zdenka Baliharova (Czech Republic), Jana Janova (Czech Republic), Michaela Lepsi (Czech Republic), Jana Blahova (Czech Republic), Jaromira Krcalova (Czech Republic), Alena Boskova (Czech Republic), Ludmila Zichova (Czech Republic), Eva Kaliskova (GSK Czech Republic), Britta Gartner (GSK Germany), Rosaleen Salter (UK), Neil Snowise (GSK UK), Miren Iturriza (HPA UK), Jim Gray (HPA UK), Celia Barberousse (formerly GSK), Liesbet De Cock (GSK Belgium).
We would also like to thank Jennifer Coward (independent freelancer for writing and editorial assistance), Uta Gomes (independent freelancer for publication coordination & editorial assistance) and Carlo Giaquinto (independent expert for assistance with data review and manuscript preparation including critical reviewing).
Rotarix is a registered trademark of the GlaxoSmithKline group of companies.
Rotateq is a registered trademark of Merck & Co.
Rotastrip is a registered trademark of Coris BioConcept.
Financial disclosure
All authors participated in the design or implementation, analysis and interpretation of the study as well as drafting and approval of the manuscript. Authors acknowledge the following potential conflicts of interest: Jean-Yves Pirçon and Nadia Meyer are employees of GSK Biologicals. Montse Soriano-Gabarró was a former employee of GSK Biologicals. Javier Diez-Domingo, Johannes Forster, Marian Patrazalek, Petr Pazdiora, Jose-Maria Baldo received consulting fees in the past 3 years. Luigi Cantarutti has no conflict of interest with GSK Biologicals.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
SPRIK Rotavirus Study Group authors
Julia Colomer (Spain), Isabel Ubeda (Spain), Trinidad Alvarez-Laviada (Spain), Mercedes Garcia (Spain), Angels Jubert (Spain), María Garcés (Spain), Carmen Peidró (Spain), Victoria Planelles (Spain), Carmen Casani (Spain), Giacomo Toffol (Italy), Silvia Carnazza (Italy), Milada Nova (Czech Republic), Lenka Prachova (Czech Republic), Dana Kabzanova (Czech Republic), Iva Kubiasova (Czech Republic), Jirina Rusinova (Czech Republic), Pavla Sandova (Czech Republic), Dagmar Valeckova (Czech Republic), Veronika Nova (Czech Republic), Johann Disselhoff (Germany), Ulrich Behre (Germany).
Rights and permissions
About this article
Cite this article
Diez-Domingo, J., Baldo, JM., Patrzalek, M. et al. Primary care-based surveillance to estimate the burden of rotavirus gastroenteritis among children aged less than 5 years in six European countries. Eur J Pediatr 170, 213–222 (2011). https://doi.org/10.1007/s00431-010-1289-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-010-1289-1