Abstract
The purpose
To analyze the outcomes of treatment and factors predicting effects of imatinib (IM) therapy in inoperable/metastatic gastrointestinal stromal tumors (GIST) CD117(+) patients.
Materials and methods
We identified 232 patients in a prospectively collected Clinical GIST Registry with advanced inoperable/metastatic GIST treated with IM 400-800 mg daily (129 males and 103 females and median age 56 years). Median follow-up time was 26 months.
Results
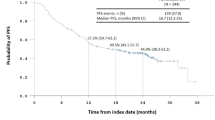
The estimated 3-year progression-free survival (PFS; calculated from the date of the start of IM) was 54% and median PFS was 40.5 months. The following factors significantly and negatively influenced PFS in univariate analysis: poor baseline World Health Organization (WHO) performance status ≥2 (P < 0.00001), tumor genotype indicating other than KIT exon 11 isoform (P = 0.005), baseline high neutrophils count (P < 0.00001), age <45 years at the diagnosis (P = 0.04), mitotic index >10/50 high-power fields (HPF) (P = 0.001), GIST histological type other than spindle-cell (P = 0.03), baseline low albumin level (P = 0.0005), low baseline hemoglobin level (P < 0.00001), and primary overtly malignant tumors (unresectable and/or metastatic lesions at presentation) (P = 0.05). We identified four factors negatively affecting PFS, statistically significant (P < 0.05) in multivariate analysis: baseline poor WHO performance status ≥2, high baseline neutrophils count (>5 × 109/l), tumor genotype indicating the presence of non-exon 11 KIT mutant and mitotic index >10/50 HPF.
Conclusions
We confirmed that many advanced GIST patients benefit from IM therapy for a prolonged time, although resistance to therapy is observed. We identified four independent biological factors influencing the PFS during long-term IM therapy.






Similar content being viewed by others
References
Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD (2005) Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under auspices of ESMO. Ann Oncol 16:566–578
Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA (2004) CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 183:1619–1628
Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal stromal tumors. J Clin Oncol 22:3813–3825
Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N, Vranckx H, Wasag B, Prenen H, Roesel J, Hagemeijer A, Van Oosterom A, Marynen P (2005) Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology 128:270–279
Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, Scurr M, Hagemeijer A, van Glabbeke M, van Oosterom AT; EORTC Soft Tissue, Bone Sarcoma Group (2004) Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40:689–695
Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I. EORTC Soft Tissue, Bone Sarcoma Group; The Italian Sarcoma Group; Australasian GastroIntestinal Trials Group (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42:1093–1103
Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G (2002) Clincial management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 33:466–477
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors. Recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Demetri GD, von Mehren M, Blanke CD, van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silvermann S, et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. NEJM 347:472–480
Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–465
Gold JS, van der Zwan SM, Gonen M, Maki RG, Singer S, Brennan MF, Antonescu CR, DeMatteo RP (2007) Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol 14:134–142
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CDM, Silberman S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342–4349
Heinrich MC, Corless CL, Demetri GD, Blanke DC, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, van den Abbele AD, Druker BJ, Kiese B, Eisenberg B, et al (2006) Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 29:4764–4774
Hirota S, Isozaki K, Moriyama Y, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580
Judson I, Ma P, Peng B, Verweij J, Racine A, di Paola ED, van Glabbeke M, Dimitrijevic S, Scurr M, Dumez H, van Oosterom A (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour, a retrospective population pharmacokinetic study over time: EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55:379–386
Lasota J, Miettinen M (2006) KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Sem Diagn Pathol 23:91–102
Le Cesne A, Van Glabbeke M, Verwiej J, Casali P, Zalcberg P, Reichardt P, Issels RD, Judson IR, Blay JY (2006) Is a stable disease according to RECIST criteria a real stable disease in GIST patients treated with imatinib mesylate (IM) included in the intergroup EORTC/ISG/AGITG trial? In: J Clin Oncol, ASCO 2006 annual meeting proceedings part I, vol 24, No. 18S (Suppl). Atlanta, p 9510
Miettinen M, Lasota J (2001) Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438:1–12
van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, Silberman S, Nielsen OS; European Organisation for Research, Treatment of Cancer Soft Tissue and Bone Sarcoma Group (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358:1421–1423
van Oosterom, Judson IR, Verwiej J, Stroobants S, Dumez H, Donato di Paola E, Sciot R, Van Glabbeke M, Dimitrijevic S, Nielsen OS; European Organisation for Research, Treatment of Cancer Soft Tissue and Bone Sarcoma Group (2002) Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 38(Suppl 5):S83–S87
Ruka W, Rutkowski P, Kaminska J, Steffen J, Rysinska A (2001) Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol 12:1423–1432
Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J (2002) Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. Int J Cancer 100:463–471
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Trent JC, Benjamin RS (2006) New developments in gastrointestinal stromal tumor. Curr Opin Oncol 18:386–395
Van Glabbeke M, Verweij J, Casali PG, Le Cesne A, Hohenberger P, Ray-Coquard I, Schlemmer M, van Oosterom AT, Goldstein D, Sciot R, Hogendoorn PC, Brown M, Bertulli R, Judson IR (2005) Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 23:5795–5804
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364:1127–1134
Wakai T, Kanda T, Hirota S, Ohashi A, Shirai Y, Hatakeyama K (2004) Late resistance to imatinib therapy in a metastatic gastrointestinal stromal tumour is associated with a second KIT mutation. Br J Cancer 90:2059–2061
Wardelmann E, Merkelbach-Bruse E, Pauls K, Thomas N, Schildhaus HU, Heinicke T, Speidel N, Pietsch T, Buettner R, Pink D, Reichardt P, Hohenberger P (2006) Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res 12:1743–1749
Acknowledgments
We thank all specialists devoted to GIST problem participating in Clinical GIST Registry; M. Rosinska, MD for statistical advice and J. Lasota, MD from Armed Forces Institute of Pathology, Washington, DC., for mutational analysis of the subset of tumors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutkowski, P., Nowecki, Z.I., Dębiec-Rychter, M. et al. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs). J Cancer Res Clin Oncol 133, 589–597 (2007). https://doi.org/10.1007/s00432-007-0202-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0202-4