Abstract
Background
Rho-like small GTPases, including RhoA, Rac1 and Cdc42, are crucial for the regulation of a large variety of biological processes such as the cytoskeletal organization and gene transcription. The activities of Rho GTPases are predominantly controlled by guanine nucleotide exchange factors (GEFs), which activate GTPases by catalyzing the exchange of bound GDP for GTP. Earlier, we have identified the Tiam1 gene as an invasion-inducing gene that encodes a specific activator (GEF) of the Rac GTPase. We found that Tiam1-mediated Rac signaling functions in various aspects of tumorigenicity including the formation and progression of Ras-induced skin tumors and Wnt-induced intestinal tumors. Here, we further distinguish the oncogenic pathways that depend on Tiam1 signaling in the mammary gland.
Material and methods
We crossed Tiam1 knockout mice with MMTV-c-myc and MMTV-c-neu transgenic mice, in which the expression of both oncogenes is targeted to the mammary gland leading to mammary tumorigenesis.
Results
We found Tiam1 important for Neu-induced tumor formation and progression but not for Myc-induced tumors. Tiam1-deficiency delayed Neu-induced tumor initiation and reduced metastasis but had no effect on the growth of the MMTV-c-neu tumors.
Conclusion
Our data indicate that the Rac activator Tiam1 contributes to tumorigenicity induced by specific oncogenic signaling pathways only.
Similar content being viewed by others
Introduction
The activity of Rho-like GTPases in response to receptor stimulation is strictly controlled to stimulate, locally and temporally, specific downstream signaling pathways in cells. The regulators of the activity of Rho GTPases consist of three classes of proteins: guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). To date, over 70 Rho GEFs have been identified (Schmidt and Hall 2002). In earlier studies, we have identified the Tiam1 gene (T-cell invasion and metastasis gene 1), which encodes a specific GEF and thus activator of the Rho-like GTPase Rac (Habets et al. 1994; Michiels et al. 1995). Rho GTPases are best characterized for their regulation of actin cytoskeleton dynamics, but they also control various other processes including apoptosis, cell proliferation and gene transcription (Bishop and Hall 2000). It is therefore not surprising that Rho GTPases and their regulators may contribute to various aspects of tumorigenicity (Malliri et al. 2002a; Sahai and Marshall 2002).
The activators of Rho GTPases not only catalyze GDP/GTP exchange but also contribute to RhoGTPase downstream signaling by connecting active GTPases to specific scaffold and effector proteins (Mertens et al. 2003; Rossman et al. 2005). Scaffold proteins that complex Tiam1 with components of specific Rac effector pathways include IB2 and spinophilin, which direct Tiam1-mediated Rac activation towards the p38 MAPK and p70S6 K cascades implicated in transcription and translation, respectively (Buchsbaum et al. 2002, 2003). Tiam1 also binds to different components of the Par polarity complex and thereby regulates polarity processes in various cell types. Tiam1 associates with Par3 and PKCξ and connects Tiam1-mediated Rac signaling to the establishment of apical–basal cell polarity in contacting epithelial cells as well as front–rear polarity in freely migrating epithelial cells (Chen and Macara 2005; Mertens et al. 2005; Pegtel et al. 2007). Tiam1 also associates with activated Rap1 and in conjunction with the Par polarity complex controls chemokine-induced T-cell polarization (Gerard et al. 2007). The association of Tiam1 with IRSp53 and p21Arc provides a direct link between Tiam1-mediated Rac activation and Arp2/3 complex-controlled actin polarization, which is required for cytoskeletal dynamics during cell migration and cell polarization (Connolly et al. 2005; ten Klooster et al. 2006). In receptor signaling, activated Ras may activate Rac by direct binding to Tiam1 (Lambert et al. 2002) or indirectly by activation of phosphoinositide 3-kinase (PI3-kinase) that may recruit and activate Tiam1 and thereby Rac downstream of Ras (Fleming et al. 2000; Zondag et al. 2000).
Tiam1 is expressed in human and rodent tumor cells of different tissue origin and has been shown to affect various aspects of tumorigenicity (Habets et al. 1995; Minard et al. 2004). The influence of Tiam1 on different stages of tumor development is illustrated in previous studies using mouse tumor model systems and Tiam1 knockout (Tiam1 −/−) mice. Tiam1-deficiency inhibits Ras-induced mouse skin tumor initiation and growth in a two-stage DMBA/TPA carcinogenesis model (Malliri et al. 2002b). These skin tumors arise by DMBA-induced Ras mutations in keratinocytes of the epidermis. Tiam1 −/− mice are more resistant to the Ras-induced skin tumor development because epidermal keratinocytes are more susceptible for Ras-induced apoptosis during tumor initiation. Although the few Ras-induced skin tumors that do occur in Tiam1 −/− mice grow much slower than wild type tumors, they convert more frequently to a malignant phenotype, presumably as a result of the function of Tiam1 in maintenance of E-cadherin-based cell–cell adhesions (Malliri et al. 2002b, 2004). Such a bifunctional effect of Tiam1-deficiency on tumor formation and progression was also found for intestinal tumors in APC Min (multiple intestinal neoplasia) mutant mice (Malliri et al. 2006). Tiam1 is a Wnt-responsive gene in colon cells and its deficiency reduces the formation and growth of polyps in APC Min mutant mice but promotes invasion of progressed malignant intestinal tumors (Malliri et al. 2006). These in vivo data indicate that Tiam1 functions downstream of at least two independent oncogenic signaling pathways, i.e., the Ras and the Wnt pathway.
Tiam1 is thus a potential therapeutic target, and chemical inhibitors have been developed to inhibit the function of Tiam1 and Rac in tumors in vivo (Gao et al. 2004; Shutes et al. 2007). In this context, it is important to decipher the specificity of Tiam1 as a modifier of tumor development and progression in the context of different oncogenic signaling pathways and of tumor cell types. Therefore, we investigated the function of Tiam1 in mammary tumorigenesis induced by two alternative oncogenic signaling pathways. We crossed Tiam1 −/− mice with two strains of breast cancer prone transgenic mice that express oncogenic Myc or Neu under the control of the mouse mammary tumor virus (MMTV) promoter. Interestingly, we found that Tiam1-deficiency did not influence Myc-induced tumorigenesis but specifically impaired c-neu induced mammary tumor formation in mice, illustrating that Tiam1-mediated Rac signaling is required for only specific oncogenic signaling pathways that lead to tumorigenesis.
Materials and methods
Mice
A congenic line of FVB/Tiam1 −/− mice was used, which was generated as described earlier (Malliri et al. 2002b). Transgenic MMTV-c-neu, line TG.NK (Muller et al. 1988) and MMTV-c-myc (Stewart et al. 1984) mice were purchased from Charles River Laboratories. All transgenic mice were on FVB background. Transgenic male mice were crossed with Tiam1 −/− females. The resulting Tiam1 ±/MMTV-oncogene males were backcrossed with female Tiam1 +/+ and Tiam1 −/− mice. From their offspring, Tiam1 +/+/MMTV-oncogene and Tiam1 ±/MMTV-oncogene males were backcrossed to Tiam1 +/+ and Tiam1 −/− females, respectively, to yield the experimental groups: Tiam1 +/+/MMTV-oncogene, Tiam1 ±/MMTV-oncogene, Tiam1 −/−/MMTV-oncogene. Only females were used for subsequent analyses. MMTV-c-neu females were kept as virgins throughout the entire observation period. MMTV-c-myc females underwent forced breeding for two pregnancies to promote tumorigenesis. Mice were monitored by palpation for tumors and killed when they harbored a mammary tumor that reached a size of 10 mm. Local ethics committee for animal experiments approved the mouse experiments according to the Dutch law that implements the European guideline 86/609/EEG.
Histology and immunohistochemistry
H&E stainings and immunohistochemistry were performed on 4 μm paraffin-embedded tissue sections as described (Strumane et al. 2005). Antibodies used are as follows: an anti-DH Tiam1-specific rabbit polyclonal antibody (Habets et al. 1994), polyclonal anti-Keratin 1 (Covance/Babco, 1:250), anti-Keratin 8 (troma-1; University of Iowa, Department of Biological Sciences, Iowa City, Iowa USA; 1:400) and anti-Keratin 14 (Covance/Babco; 1:10,000).
Whole mounts of mammary glands
Inguinal mammary fat pads were excised from euthanized mice and stretched on a glass slide for fixation in methanol:chloroform:acidic acid (6:3:1) for at least 24 h. After washing in 70% ethanol for 1 h, the slides were rinsed in water and stained in carmine for 24 h. All incubations were performed at room temperature (RT). The fat pads were dehydrated in a graded series of alcohols and kept in methyl salicylate to make photographic images.
Isolation mammary epithelial cells
The left and right mammary fat pads 2 and 3 were excised from euthanized mice at 16 weeks of age. The isolated tissues were washed three times in 70% ethanol and transferred to L15 medium (Gibco BRL) with 10% fetal calf serum (FCS). The fat pads were chopped using a scalpel and digested in a 0.3% collagenase–0.15% trypsin mix in serum-free L15 medium at 37°C for 1 h with periodic shaking. Cells were pelleted and washed four times with L15 medium with serum and subsequently incubated in DMEM containing 10% FCS, 2 mM l-glutamine (l-Gln) and 100 U/ml penicillin/100 μg/ml streptomycin (P/S) at 37°C, 5% CO2, 5% O2 to remove contaminating fibroblasts. After 1 h, the epithelial cells were still floating in the medium and could be easily separated from the fibroblasts that had attached to the bottom of the culture flasks. The pelleted epithelial cells were resuspended and cultured for maximal 3 days in DMEM:F12 (1:1; Gibco-BRL) medium containing 10% FCS and P/S and supplemented with 5 μg/ml insulin, 5 ng/ml choleratoxin and 5 ng/ml EGF.
Western blotting
Lysates were prepared using standard SDS or RIPA lysis buffers as indicated. Cultured cells were washed with PBS and scraped in lysis buffer. Snap frozen tumor material was first grinded in a mortar and than lysed. Proteins were separated by SDS-PAGE and transferred to Immobilon-P membrane (Millipore). After blocking with 5% skimmed milk, the blots are probed using the indicated antibodies. Primary antibodies used were anti-DH [(Habets et al. 1994);1/500] and C-16 (sc-872, Santa Cruz, Venendaal, The Netherlands; 1/1.000) against Tiam1, anti-α-tubulin (Clone B-5-1-2, Sigma, 1/5.000), anti-c-erbB2/HER-2/neu (Ab-17, Neomarkers, 1/2.000) and anti-Rac1 (clone 23A8, Upstate Biotechnology, Venendaal, The Netherlands; 1/1.000). As secondary antibodies, peroxidase-conjugated IgGs were used followed by enhanced chemiluminiscence (ECL) detection (Amersham).
Apoptosis quantification
MDA-MB-361 cells were cultured in DMEM:F12 (1:1; Gibco-BRL) medium supplemented with P/S, 10% FCS, 10 ng/ml EGF and 10 μg/ml insulin. The Tiam1-specific siRNA oligo GCGAAGGAGCAGGTTTTCT (Malliri et al. 2004) was transfected into the MDA-MB-361 cells using the Dharmafect-1 reagent (Dharmacon). A scrambled sequence was used as nonspecific control siRNA oligo (siCONTROL nontargeting siRNA, Dharmacon). Six hours after transfection, the transfection mix was replaced by culture medium, which was refreshed again 24 h after transfection and apoptosis was quantified 72 h after transfection. Both floating and adherent cells were lysed together and apoptosis was analyzed using the Cell Death Detection ELISA kit (Roche) according to manufactures instructions.
Results
Tiam1 expression in the mammary gland is increased in tumor tissue
We started our analyses by determining Tiam1 expression in the mammary glands and mammary tumors of transgenic and nontransgenic female mice. Tiam1 is expressed in the normal mammary gland as shown by Western blot analysis of primary cells isolated from 16-week-old wild type mice (Fig. 1a, left lane). At this age, we found increased Tiam1 expression levels in the precancerous mammary gland of MMTV-c-neu transgenic mice (Fig. 1a, right lane). Western blot analysis revealed Tiam1 expression in both MMTV-c-myc and MMTV-c-neu tumors (Fig. 1b). Consistent with these data, others also found that Tiam1 was increased in MMTV-c-neu tumors compared to normal tissue at the mRNA level (Landis et al. 2005). Tiam1 expression in tumors was confirmed by immunohistochemical staining of mammary tumors isolated from Tiam1 +/+ mice (Fig. 1c). These data demonstrate that Tiam1 is expressed in the mammary gland and that its expression is increased in Neu-induced and Myc-induced mammary tumors.
Tiam1 expression in mouse mammary tissue and tumors. a Tiam1 is detected (C16 antibody) by Western blotting in primary epithelial cells isolated from precancerous mammary glands of 16-week-old mice. Tiam1 expression is increased in the mammary gland of MMTV-c-neu mice compared to wild type mice. The same blot was probed for the detection of α-tubulin for loading control. b Western blot analysis of Tiam1 protein levels in tumor samples derived from MMTV-c-myc and MMTV-c-neu mice (C16 antibody). Two SDS lysates of snap-frozen tumors isolated from different mice are shown for each transgenic line. The same blot was probed with a Rac antibody and used as a loading control. c Expression of Tiam1 in histological tumor slides. Mammary carcinomas from MMTV-c-myc;Tiam1 +/+ and MMTV-c-neu;Tiam1 +/+ are shown probed for Tiam1 (anti-DH antibody). The scale bar indicates 100 μm
Normal mammary gland development in Tiam1-deficient mice
Tiam1 knockout mice are viable and do not show any obvious aberrant phenotype (Malliri et al. 2002b). Also the mammary glands of Tiam1 −/− mice are functionally normal as Tiam1 −/− females are able to suckle their offspring. To exclude that tumorigenesis is influenced by morphological differences in mammary gland development, we compared in detail the appearance of the inguinal mammary glands of virgin Tiam1 −/− female mice with that of Tiam1 +/+ mice in nontransgenic and transgenic animals at different ages (Fig. 2). The appearance of Tiam1 −/− mammary glands was identical to that of wild type glands in adult mice (not shown). However, we found a delay in the early development of the mammary gland in Tiam1 −/− when compared to wild type mice. The outgrowth of the inguinal mammary glands is determined by how far the glands extend beyond the inguinal lymph node (to the right side in Fig. 2). The mammary ductal system was significantly less far proliferated within the adipose stroma in 4-week-old and 6-week-old Tiam1 −/− mice when compared to age-matched wild type mice (Fig. 2 left panel). At 8 weeks, the appearance of the mammary gland in Tiam1+/+ and Tiam1−/− mice was indistinguishable, indicating that development was delayed but not impaired (Fig. 2 left panel). This delay in early mammary gland elongation during puberty in Tiam1 −/− mice was also observed for both transgenic strains in a Tiam1 −/− background as exemplified in 6-week-old mice (Fig. 2 right panel). By analyzing mammary glands at different time points, we found that the delay in outgrowth of the mammary gland was most apparent at 4 weeks of age, declined within the subsequent weeks and by 8 weeks of age we could not discriminate anymore between wild type and Tiam1 knockout mammary glands. As the first tumors in MMTV-oncogene mice became detectable only by 17 weeks of age, we conclude that it is unlikely that the delay in early normal mammary gland development affects mammary tumorigenesis in the mouse mammary tumor models used.
Analysis of mammary gland development. Inguinal mammary glands from 4-, 6-, and 8-week-old Tiam1 +/+ and Tiam1 −/− virgin female mice were compared within the nontransgenic mice (left panel) and from 6-week-old Tiam1 +/+ and Tiam1 −/− mice in the MMTV-c-myc and MMTV-c-neu transgenics (right panel). All mammary glands are positioned in the same orientation so that the outgrowth of the mammary glands is presented from left to right. The distance towards or beyond the inguinal lymph node is a measure for the outgrowth of the mammary gland. Representative images show the delay in elongation of the system of mammary ducts in the Tiam1 −/− mice compared to Tiam1 +/+ mice in the different strains. Mammary whole mounts were stained with carmine red. The scale bar indicates 1.5 mm
Tiam1 is involved in mammary tumorigenesis induced by Neu and not by Myc
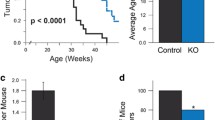
To address whether Tiam1 is involved in specific oncogenic pathways, we examined mammary tumorigenesis in MMTV-c-neu and MMTV-c-myc female mice in Tiam1 +/+ and Tiam1 −/− backgrounds. Weekly palpation of the mice revealed that Tiam1-deficiency strongly delayed the appearance of tumors in MMTV-c-neu transgenic mice, whereas the latency of mammary tumors induced by Myc was not affected by the loss of Tiam1 (Fig. 3). MMTV-c-myc mice developed mammary tumors with the same latency in Tiam1 +/+ and Tiam1 −/− mice (Fig. 3a). Although all except one of the MMTV-c-neu mice developed palpable tumors over an observation period of 1 year (Table 1), the latency was significantly longer in the Tiam1-deficient mice (P < 0.001) (Fig. 3b). At the time when tumors were detected in 100% of the MMTV-c-neu;Tiam1+/+ mice (age 29 weeks), detectable tumors were found in only 19% of the MMTV-c-neu;Tiam1−/− mice. This delay in the rate of tumor initiation is also reflected in the T 50 that denotes the age at which 50% of the populations possess at least one palpable tumor. The T 50 was 23.5 weeks for MMTV-c-neu;Tiam1+/+ mice and 32.5 weeks for MMTV-c-neu;Tiam1−/− mice. Together, these data indicate that, while Tiam1 is not required for the induction of mammary tumors by Myc, it does play a critical role in the initiation of mammary tumors induced by Neu. More specifically, Tiam1-deficiency extends the latency of Neu-induced mammary tumor initiation.
Kinetics of oncogene-induced mammary tumor initiation in Tiam1 +/+ and Tiam1 −/− mice. The age when a palpable mammary tumor first appears represents the latency of tumor initiation. a Tumor initiation in MMTV-myc;Tiam1 −/− (n = 20) compared to MMTV-myc;Tiam1 +/+ (n = 29) is not different (P = 0.41, Student’s t test). b The longer latency in MMTV-neu;Tiam1 −/− (n = 21) compared to MMTV-neu;Tiam1 +/+ (n = 26) is statistically significant (P < 0.001, Student’s t test). N is the number of mice analyzed
Tiam1-deficiency does not influence the type of tumor differentiation in MMTV-c-neu and MMTV-c-myc mice
We analyzed a possible relation between the involvement of Tiam1 and the tumor characteristics. The MMTV-c-myc and c-neu tumors were defined as adenocarcinomas based on histopathological analysis of hematoxylin and eosin (H&E) stainings (Fig. 4a). To further analyze the differentiation status of the tumors in our analysis, we performed additional stainings for Keratins. The MMTV-c-myc and c-Neu tumors were all positive for Keratin 8 in both Tiam1 +/+ and Tiam1 −/− mice (Fig. 4b, A–D), indicating that they all had a glandular character, although this was less evidently suggested by the H&E staining in the case of the MMTV-c-neu tumors. Keratin 1 is normally not expressed in the mammary gland and is used as a marker for epidermal differentiation in mammary tumors. All tumors were negative for Keratin 1 (Fig. 4b, E–H), while the adjacent skin tissue served as an intrinsic positive control (not shown). Although the Keratin 1 staining suggested that none of the tumors contained epidermal characteristics, MMTV-c-myc tumors showed a positive Keratin 14 staining indicating squamous metaplasia (Fig. 4b, I, J) in both Tiam1 +/+ and Tiam1 −/− mice. However, the MMTV-c-neu tumors were completely negative for Keratin 14 (Fig. 4b, K, L). From these immunohistological analyses, we conclude that the presence or lack of Tiam1 has no effect on the differentiation type of the MMTV-c-myc and MMTV-c-neu mammary tumors. The MMTV-c-neu tumors show homogeneously a glandular differentiation (Keratin 8-positive), whereas MMTV-c-myc tumors consist of different components with glandular (Keratin 8-positive) and squamous (Keratin 14 positive) differentiation. This is in agreement with the notion that MMTV-c-neu mice uniformly develop mammary tumors (Bargmann et al. 1986; Muller et al. 1988), whereas MMTV-c-myc mice develop mammary tumors in a stochastic fashion. As reported earlier, c-myc is necessary but not sufficient for tumorigenesis, indicating that additional events are required for mammary tumor development in these mice (Li et al. 2000; Stewart et al. 1984; Tsukamoto et al. 1988). Taken together, MMTV-c-neu tumors can be discriminated from MMTV-c-myc tumors by the fact that they show glandular differentiation only and no squamous characteristics. Furthermore, the presence or absence of Tiam1 does not influence the histological type of tumors raised by Myc or Neu expression.
Tiam1 does not affect differentiation of mammary tumors in MMTV-c-myc and MMTV-c-neu mice. a Sections of mammary tumors from the indicated transgenic mice, stained with hematoxylin and eosin (H&E). The scale bar indicates 100 μm. b Immunohistochemical stainings of MMTV-c-myc and MMTV-c-neu tumors in Tiam +/+ and Tiam1 −/− mice. All tumors are positive for Keratin 8, which is specific for glandular characteristics. All tumors are negative for the epidermal marker Keratin 1. Keratin 14 staining shows squamous differentiation in MMTV-c-myc tumors, while MMTV-c-neu tumors are negative for Keratin 14. The scale bar indicates 100 μm
Tiam1-deficiency affects initiation but not growth of neu-induced mammary tumors
Western blot analysis of mammary tumors in Tiam1 +/+ and Tiam1 −/− mice showed that disruption of Tiam1 expression did not alter the expression levels of the Neu protein (Fig. 5a). This excludes the possibility that the delay in initiation of Neu-induced mammary tumors in Tiam1 −/− mice was caused by an inadequate expression of the neu transgene in the mammary glands of these mice. The latency of MMTV-c-neu-induced tumors in the Tiam1 ± group was intermediate between that in the Tiam1 +/+ and Tiam1 −/− groups (Fig. 5b) suggesting a dose-dependent effect of Tiam1 on the initiation of Neu-induced mammary tumors. We found earlier a similar dose-dependent effect of Tiam1 on Ras-induced skin tumors (Malliri et al. 2002b).
Tiam1 affects number of neu-induced mammary tumors. a Western blot analyses. SDS lysates of snap frozen tumors isolated from Tiam1 +/+ and Tiam1 −/− MMTV-c-neu mice were blotted and probed for detection of endogenous Tiam1 and transgenic Neu. The same blot was probed for Rac1 and used as loading control. b Kinetics of the tumor susceptibility in MMTV-neu;Tiam1 ± mice (N = 20) is intermediate between MMTV-neu;Tiam1 +/+ (N = 26) and MMTV-neu;Tiam1 −/− (N = 21) mice. The percentage of mice with a palpable mammary tumor is presented in time. N is the number of mice analyzed. c Plot of the total number of tumors with a size >4 mm per mouse in MMTV-c-neu;Tiam1 −/−, MMTV-c-neu;Tiam1 ± and MMTV-c-neu;Tiam1 +/+ mice at the time of necropsy. The median number of tumors per mouse is indicated as a horizontal line and is 4 in Tiam1 +/+ mice (N = 23; range 1–15 tumors per mouse), 3 in Tiam1 ± mice (N = 15; range 1–8 tumors per mouse) and only 2 in Tiam1 −/− mice (N = 17; range 1–7 tumors per mouse). N is the number of mice analyzed. d Tumor growth is similar in Tiam1 +/+ and Tiam1 −/− mice. The average time for a tumor to grow out from just palpable to a diameter that reached 10 mm in the Tiam1 −/− mice (N = 14) compared to Tiam1 −/− mice (N = 23) is not significantly changed (P = 0.21, Student’s t test). N is the number of mice analyzed
The decreased susceptibility to Neu-induced tumors in Tiam1-deficient mice was also reflected by a decreased number of tumors per mouse (Fig. 5c). Mice were euthanized for dissection when they harbored at least one mammary tumor that reached a size of 10 mm. The median number of tumors with a size >4 mm per mouse is four in Tiam1 +/+ mice, three tumors per mouse in Tiam1 ± and two tumors per mouse in Tiam1 −/− mice, again suggesting a dose-dependent effect of Tiam1 on tumor initiation (Fig. 5c). Tumor growth was determined by the time between detection of the first palpable tumor and necropsy, i.e., when the tumor had grown out to a size that reached 10 mm. We found that the average growth of individual MMTV-c-neu tumors was not significantly different in Tiam1 +/+ and Tiam1 −/− backgrounds (Fig. 5d). This indicates that once a tumor is initiated in the Tiam1 −/− mice, it is able to grow as fast as in the Tiam1 +/+ mice. Tiam1-deficiency also did not affect mammary tumor growth in the MMTV-c-myc model in which tumor initiation was not affected (not shown). Together, these data indicate that the delayed latency and the fewer tumors in Tiam1 −/− mice compared to Tiam1 +/+ mice are a consequence of a specific function of Tiam1 in Neu-induced tumor initiation rather than an involvement of Tiam1 in the growth of the mammary tumors.
Tiam1 provides survival signaling in Neu-induced mammary tumor cells
Prevention of apoptosis is a necessary step during the process of tumor initiation. The increased susceptibility for apoptosis was found to be the underlying mechanism of decreased skin tumor incidence in Tiam1 −/− mice (Malliri et al. 2002b). As in Ras-induced skin tumors, it is possible that Tiam1-deficiency results in a reduced number of tumors in the MMTV-c-neu mouse model by increasing the susceptibility to apoptosis of the targeted mammary epithelial cells. Attempts to analyze apoptosis in vivo in established tumors did not discriminate Neu-induced tumors between Tiam1+/+ and Tiam1−/− mice. Therefore, we analyzed the dependency of the survival of Neu-expressing breast cancer cells on Tiam1 in vitro. As a model, we used the human breast cancer cells MDA-MB-361, which are characterized by high Neu expression. Western blot analysis showed that MDA-MB-361 cells express Tiam1 that can be downregulated by Tiam1-specific siRNA (Fig. 6a). The susceptibility to apoptosis was measured using a cell death detection kit that quantitatively determines cytoplasmic histone-associated DNA fragments by ELISA (enzyme-linked immunosorbent assay). Interestingly, the number of apoptotic cells is increased upon downregulation of Tiam1 when compared to cells that were transfected with a nonspecific control siRNA (Fig. 6b), indicating that Tiam1 also controls apoptosis in Neu-expressing tumor cells.
a, b Apoptosis downstream of Neu signaling is Tiam1-dependent. a Western blot analysis showing Tiam1 downregulation in MDA–MB-361 breast cancer cells 3 days after transfection of Tiam1-specific siRNA. As a control, a nonspecific scrambled siRNA sequence was used. The same blot was probed with a Rac antibody and used as a loading control. b Tiam1 downregulation increased apoptosis as determined by the measurement of the amount of cytoplasmic histone-associated DNA fragments. Apoptosis was measured 3 days after siRNA transfection. Bars show the average of three independent experiments. c–e Metastasis of Neu-induced tumors was lower in Tiam −/− than in Tiam +/+ mice. c Representative image of an H&E-stained slide showing a typical nonextravasating metastatic embolus of mammary adenocarcinoma cells in the lung of a MMTV-c-neu mice. The scale bar indicates 100 μm. d Mammary tumor-bearing Tiam1 +/+ mice show pulmonary metastases in 50% of the animals (N = 22), while metastatic emboli in the lungs were found for only 18% of the Tiam −/− mice (N = 17). N is the number of mice analyzed. e E-cadherin expression in mammary tumors in MMTV-c-neu;Tiam1 +/+ and MMTV-c-neu;Tiam1 −/− mice. The scale bar indicates 100 μm
Tiam1-deficiency affects metastatic potential of Neu-induced mammary tumors
We also analyzed the MMTV-c-neu tumor-bearing mice for the presence of metastases by histopathological analysis. We found metastases mainly in the lungs and occasionally in the heart. This is consistent with earlier observations that MMTV-c-neu-induced mammary tumors metastasize to the lung with high frequency (Guy et al. 1992; Siegel et al. 2003; Taverna et al. 2005). Most of the observed lung lesions were intravascular metastases representing tumor cells that remain fully contained within a pulmonary vessel without extravasations as shown by an H&E staining of lung tissue (Fig. 6c). The percentage of mice with lung micrometastases was found to be significantly lower in MMTV-c-neu;Tiam1 −/− mice compared to MMTV-c-neu;Tiam1 +/+ mice. We detected lung metastases in 50% of the MMTV-c-neu;Tiam1 +/+ mice and only in 18% of the MMTV-c-neu;Tiam1 −/− mice (Fig. 6d). Tiam1 has been shown to be involved in strengthening of E-cadherin-based cell–cell adhesions, which influences metastatic capacities of tumor cells (Malliri et al. 2004). However, E-cadherin staining of MMTV-c-neu mammary tumors showed fields of E-cadherin-negative cells within a majority of E-cadherin-positive tumor cells in both Tiam1 +/+ and Tiam1 −/− mice (Fig. 6e), suggesting that Tiam1-deficiency did not affect the metastatic capacity of Neu-induced mammary tumors by affecting the E-cadherin-mediated cell–cell adhesions. Presumably, the lower incidence of metastases in the Tiam1 −/− mice is a direct consequence of the lower number of mammary tumors produced per mouse in Tiam1 −/− mice.
Discussion
We crossed Tiam1 −/− mice with MMTV-c-myc and MMTV-c-neu transgenic mice to study the consequences of Tiam1-deficiency in Myc-induced and Neu-induced mammary tumorigenesis. Our analyses revealed that Tiam1 is required for oncogenic signaling induced by Neu but not by Myc. More specifically, we found that Tiam1-deficiency delays the initiation of Neu-induced mammary tumors but does not affect the growth of these tumors. Initiation and growth of Myc-induced mammary tumors was independent of the expression of Tiam1.
Besides the dramatic delay in the onset of the first detectable Neu-induced mammary tumors, also the total number of tumors per mouse was lower in Tiam1 −/− mice than in Tiam1 +/+ animals. This is consistent with the findings in skin and intestinal tumors, where the latency of tumor onset and the number of tumors per mouse were dramatically decreased in a Tiam1-deficient background (Malliri et al. 2002b, 2006). In the skin tumor model, we found that Tiam1 −/− mice produced less Ras-induced tumors, because keratinocytes in the basal layer of the epidermis are more susceptible to apoptosis (Malliri et al. 2002b). Recently, we found that the Tiam1/Rac-mediated survival pathway in keratinocytes acts through ROS-mediated activation of the ERK pathway (Rygiel et al. 2008). Also in APC Min mice, the decreased initiation of intestinal tumors by aberrant β-catenin signaling in Tiam1-deficient mice compared to wild type mice was attributed to increased apoptosis susceptibility (Malliri et al. 2006). Consistent with this, Tiam1 expression levels correlate with apoptosis susceptibility in human colon tumor cells (Minard et al. 2006).
It is likely that Tiam1-deficiency in the MMTV-c-neu model affects tumor initiation by increasing the susceptibility to apoptosis of the targeted mammary epithelial cells. We have attempted to study apoptosis sensitivity in vivo, but we could not find significant differences in apoptosis between established Neu-induced tumors produced in Tiam1 +/+ and Tiam1 −/− mice (data not shown). Analysis of apoptosis in tumor samples of MMTV-c-neu tumors by TUNEL and Caspase 3 stainings appeared difficult because of the heterogeneity of these tumors. Moreover, apoptosis resistance is most likely essential during the initiating events of tumorigenicity, which is difficult to study in vivo. Once tumors have been formed, differences in apoptosis sensitivity in Tiam1 +/+ and Tiam1 −/− tumors are presumably not detectable anymore, as Tiam1-independent events have rescued the tumor-initiating cells from apoptosis. We performed therefore in vitro studies using MDA-MB-361 breast cancer cells with high Neu expression and found that the survival signaling of these tumor cells is dependent on the presence of Tiam1. Similarly, as found in DMBA-induced skin tumors and β-catenin-induced intestinal tumors, the presence of Tiam1 seems to be required to prevent apoptosis during initiation of mammary tumors by the Neu oncogene. Tiam1-mediated Rac-signaling might prevent apoptosis by activating various well-known survival signaling pathways including the NFkappaB and ERK pathways (Joneson and Bar-Sagi 1999; Rygiel et al. 2008; Zahir et al. 2003).
Although less mammary tumors were produced in Tiam1 −/− mice, the growth of the tumors in Tiam1 +/+ and Tiam1 −/− mice was the same once they were formed. In contrast, we showed in skin and intestinal tumors a function of Tiam1 in both initiation and growth of these tumors (Malliri et al. 2002b, 2006). Interestingly, in DMBA (Ras)-induced skin tumors, decreased growth of tumors in Tiam1 −/− mice was found only when proliferation of tumors was promoted by TPA treatment. Skin tumors that were generated by treatment with DMBA only grew equally well in the presence or absence of Tiam1 (Malliri et al. 2002b), indicating that TPA-induced but not DMBA-induced proliferation depends on Tiam1-mediated Rac activation. TPA is able to induce cyclin D1 expression, a regulator of cell proliferation (Yan and Wenner 2001), suggesting that Tiam1 is required for TPA-induced proliferation by influencing cyclin D1 levels. Moreover, both the Ras and Neu oncogenes are absolutely dependent on cyclin D1 expression for mammary tumor formation in MMTV-ras and MMTV-c-neu transgenic mice (Yu et al. 2001). Cyclin D1-deficient mice are resistant to Ras-induced and Neu-induced mammary tumors, while they remain fully sensitive to other oncogenic pathways (Yu et al. 2001). However, the fact that the proliferation of Neu-induced tumors is independent of Tiam1 suggests that Tiam1 does not regulate cyclin D1 levels in mammary tumors. Indeed, tumor lysates from MMTV-c-neu;Tiam1 +/+ and MMTV-c-neu;Tiam1 −/− mice revealed a large variation in cyclin D1 levels independent of the presence of Tiam1 (data not shown). As Neu predominantly signals through Ras and the growth of DMBA-only treated skin tumors is independent of Tiam1, it is unlikely that Tiam1–Rac signaling contributes to Ras-controlled proliferation of tumors. Interestingly, it has been shown that Neu-mediated protection from apoptosis is dependent on its association with the Par polarity complex, while Neu-mediated proliferation is not (Aranda et al. 2006). Tiam1 associates with the Par polarity complex and is able to activate this complex (Mertens et al. 2006), providing a possible mechanism by which Tiam1 could interfere in initiation but not growth of Neu-induced mammary tumors.
A higher number of metastases was found in the MMTV-c-neu;Tiam1 +/+ mice when compared to MMTV-c-neu;Tiam1 −/− mice, suggesting that Tiam1 promotes metastasis of breast tumors. Studies in human tumors also show a positive correlation between Tiam1 expression and progression and invasiveness of mammary, colon and prostate tumors (Adam et al. 2001; Engers et al. 2006; Liu et al. 2007; Minard et al. 2005, 2006). This is in contrast to the findings in skin and intestinal tumors, where progression was associated with loss of Tiam1 (Malliri et al. 2002b, 2006). The latter could be explained by a function of Tiam1 in the formation and maintenance of intercellular adhesions (Engers et al. 2001; Hordijk et al. 1997; Mertens et al. 2005; Uhlenbrock et al. 2004). In the MMTV-c-neu mice, the metastases appear in pulmonary blood vessels as tight tumor emboli that are thought to arise from circulating cell clumps that get stocked in the veins of the lungs. As in human inflammatory breast cancers (IBC), such circulating tumor emboli might benefit from strong E-cadherin-mediated cell–cell interactions favoring passive dissemination in distinct organs (Kleer et al. 2001; Tomlinson et al. 2001). However, we could not find significant differences in E-cadherin expression between Neu-induced tumors in Tiam1 +/+ and Tiam1 −/− mice that could support such a mechanism. Alternatively, the higher number of metastases found in the MMTV-c-neu;Tiam1 +/+ mice could be the result of the increased number of tumors found in these mice.
In conclusion, the effects of Tiam1-mediated Rac signaling on tumorigenesis appear oncogene-dependent and tumor cell type-dependent and either positively or negatively correlate with tumor progression. As Tiam1-mediated Rac activation controls different signaling pathways that may influence initiation, growth and progression of tumors, the cellular outcome of altered Tiam1 expression may depend on a balance between factors that promote or inhibit the formation and progression of tumors.
References
Adam L, Vadlamudi RK, McCrea P, Kumar R (2001) Tiam1 overexpression potentiates heregulin-induced lymphoid enhancer factor-1/beta -catenin nuclear signaling in breast cancer cells by modulating the intercellular stability. J Biol Chem 276:28443–28450
Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK (2006) Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8:1235–1245
Bargmann CI, Hung MC, Weinberg RA (1986) The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319:226–230
Bishop AL, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348(Pt 2):241–255
Buchsbaum RJ, Connolly BA, Feig LA (2002) Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol 22:4073–4085
Buchsbaum RJ, Connolly BA, Feig LA (2003) Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J Biol Chem 278:18833–18841
Chen X, Macara IG (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7:262–269
Connolly BA, Rice J, Feig LA, Buchsbaum RJ (2005) Tiam1-IRSp53 complex formation directs specificity of rac-mediated actin cytoskeleton regulation. Mol Cell Biol 25:4602–4614
Engers R, Springer E, Michiels F, Collard JG, Gabbert HE (2001) Rac affects invasion of human renal cell carcinomas by up-regulating tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 expression. J Biol Chem 276:41889–41897
Engers R, Mueller M, Walter A, Collard JG, Willers R, Gabbert HE (2006) Prognostic relevance of Tiam1 protein expression in prostate carcinomas 24. Br J Cancer 95:1081–1086
Fleming IN, Gray A, Downes CP (2000) Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J 351:173–182
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101:7618–7623
Gerard A, Mertens AE, van der Kammen RA, Collard JG (2007) The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J Cell Biol 176:863–875
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89:10578–10582
Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG (1994) Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77:537–549
Habets GG, van der Kammen RA, Stam JC, Michiels F, Collard JG (1995) Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene 10:1371–1376
Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG (1997) Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278:1464–1466
Joneson T, Bar-Sagi D (1999) Suppression of Ras-induced apoptosis by the Rac GTPase 1. Mol Cell Biol 19:5892–5901
Kleer CG, van Golen KL, Braun T, Merajver SD (2001) Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol 14:458–464
Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ (2002) Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol 4:621–625
Landis MD, Seachrist DD, Montanez-Wiscovich ME, Danielpour D, Keri RA (2005) Gene expression profiling of cancer progression reveals intrinsic regulation of transforming growth factor-beta signaling in ErbB2/Neu-induced tumors from transgenic mice. Oncogene 24:5173–5190
Li Y, Hively WP, Varmus HE (2000) Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene 19:1002–1009
Liu L, Zhao L, Zhang Y, Zhang Q, Ding Y (2007) Proteomic analysis of Tiam1-mediated metastasis in colorectal cancer 11. Cell Biol Int 31:805–814
Malliri A, ten Klooster JP, Olivio C, Collard JG (2002a) Determination of the activity of Rho-like GTPases in cells. Methods Mol Biol 189:99–109
Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG (2002b) Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature 417:867–871
Malliri A, van Es S, Huveneers S, Collard JG (2004) The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem 279:30092–30098
Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG (2006) The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J Biol Chem 281:543–548
Mertens AE, Roovers RC, Collard JG (2003) Regulation of Tiam1-Rac signalling. FEBS Lett 546:11–16
Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG (2005) The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 170:1029–1037
Mertens AE, Pegtel DM, Collard JG (2006) Tiam1 takes PARt in cell polarity 3. Trends Cell Biol 16:308–316
Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG (1995) A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375:338–340
Minard ME, Kim LS, Price JE, Gallick GE (2004) The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res Treat 84:21–32
Minard ME, Herynk MH, Collard JG, Gallick GE (2005) The guanine nucleotide exchange factor Tiam1 increases colon carcinoma growth at metastatic sites in an orthotopic nude mouse model. Oncogene 24:2568–2573
Minard ME, Ellis LM, Gallick GE (2006) Tiam1 regulates cell adhesion, migration and apoptosis in colon tumor cells. Clin Exp Metastasis 23:301–313
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P (1988) Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54:105–115
Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG (2007) The par-tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 17:1623–1634
Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180
Rygiel TP, Mertens AE, Strumane K, van der Kammen R, Collard JG (2008) The Rac activator Tiam1 prevents keratinocyte apoptosis by controlling ROS-mediated ERK phosphorylation. J Cell Sci 121:1183–1192
Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nat Rev Cancer 2:133–142
Schmidt A, Hall A (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16:1587–1609
Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ (2007) Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of rac family small GTPases. J Biol Chem 282(49):35666–35678
Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J (2003) Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA 100:8430–8435
Stewart TA, Pattengale PK, Leder P (1984) Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38:627–637
Strumane K, Rygiel TP, Collard JG (2005) The rac activator tiam1 and ras-induced oncogenesis. Methods Enzymol 407:269–281
Taverna D, Crowley D, Connolly M, Bronson RT, Hynes RO (2005) A direct test of potential roles for beta3 and beta5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res 65:10324–10329
ten Klooster JP, Evers EE, Janssen L, Machesky LM, Michiels F, Hordijk P, Collard JG (2006) Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem J 397:39–45
Tomlinson JS, Alpaugh ML, Barsky SH (2001) An intact overexpressed E-cadherin/alpha, beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res 61:5231–5241
Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE (1988) Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619–625
Uhlenbrock K, Eberth A, Herbrand U, Daryab N, Stege P, Meier F, Friedl P, Collard JG, Ahmadian MR (2004) The RacGEF Tiam1 inhibits migration and invasion of metastatic melanoma via a novel adhesive mechanism. J Cell Sci 117:4863–4871
Yan S, Wenner CE (2001) Modulation of cyclin D1 and its signaling components by the phorbol ester TPA and the tyrosine phosphatase inhibitor vanadate. J Cell Physiol 186:338–349
Yu Q, Geng Y, Sicinski P (2001) Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017–1021
Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM (2003) Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J Cell Biol 163:1397–1407
Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG (2000) Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol 149:775–782
Acknowledgments
We would like to thank the coworkers of the Division of Animal Pathology of the Netherlands Cancer Institute for technical assistance. This work was supported by the Sixth Framework Program of the European Union (BRECOSM) and by grants from the Dutch Cancer Society to J. G. Collard.
Conflict of interest statement
There is no conflict of interest regarding this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Strumane, K., Rygiel, T., van der Valk, M. et al. Tiam1-deficiency impairs mammary tumor formation in MMTV-c-neu but not in MMTV-c-myc mice. J Cancer Res Clin Oncol 135, 69–80 (2009). https://doi.org/10.1007/s00432-008-0437-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0437-8