Abstract
Purpose
DYX1C1 has three alternatively spliced transcripts. Therefore, we expect that alternative transcripts of DYX1C1 are used as a biomarker to detect specific cancer.
Methods
RT-PCR analysis is conducted in order to detect expression of the DYX1C1 gene and the PCR products were analyzed using the Image J program to compare the expression levels of each transcript.
Results
We found one of the transcripts was directly associated with an HERV-H LTR element that could be translated into protein sequence. Four new alternative transcripts were identified by RT-PCR analysis with various human tissue samples including 10 normal and adjacent tumor tissue sets. Semi-quantitative RT-PCR analysis showed the transcriptional activity of V3 and V2 was higher in tumor than in normal tissue samples, especially in the colorectal tissue samples.
Conclusion
Our results indicated that alternatively spliced transcript variants of the DYX1C1 gene could be used as cancer biomarkers to detect colorectal cancer.
Similar content being viewed by others
Introduction
DYX1C1 is a recently identified candidate gene for dyslexia. Disruption of the gene by a translocation was detected in dyslexia patients (Taipale et al. 2003; McGrath et al. 2006). The DYX1C1 gene maps to chromosome 15q21 and consists of 10 exons dispersed over about 78 kb of genomic DNA. The protein sequence encoded by DYX1C1 is 420 aa in length and the protein sequences of the nonhuman primate homolog are 98.6–99.5% similar to that of humans (Taipale et al. 2003). DYX1C1 is expressed broadly in several adult tissues including lung, kidney, and brain (Taipale et al. 2003; Wang et al. 2006), where its protein is localized in white matter including the nuclei of cortical neurons and glial cells (Taipale et al. 2003; Fisher and Francks 2006). Eight single nucleotide polymorphisms (SNPs) in the DYXICI genes of 20 patients with reading disabilities were detected (Taipale et al. 2003; McGrath et al. 2006). The 1249G-to-T and -3G-to-A mutations were among the SNPs found more frequently in patients with dyslexia than in controls (Fisher and Francks 2006). Developmental dyslexia is associated with migration anomalies in the neocortex as well genetic susceptibility (Pennington and Smith 1983; Galaburda et al. 1985; Smith et al. 1998; Chang et al. 2005; Sokol et al. 2006). DYX1C1 plays a role in the migration of neocortical neurons. Specifically it is required for the transition of the multipolar stage of migration (Wang et al. 2006).
The identification of cancer biomarkers has been a major focus of investigation in the field of cancer research. However, effective and reproducible cancer biomarker developments are still far off and enormous efforts are needed in this area (Brinkman 2004). In cancerous cells, splicing mechanisms are significantly altered by defects in splice sites caused by frequent point mutations (Venables 2004; Skotheim and Nees 2007). Aberrant neuronal-specific splicing of amphiphysin 2 in non-neuronal cells may contribute to the progression human melanoma (Ge et al. 1999). Expression levels of alternatively spliced variants of survivin are not equal to each other in malignant breast tissues. In the case of breast cancer, different survivin transcripts show different functions in the progression of apoptosis (Ryan et al. 2005; Pajares et al. 2007). Our study focused on the application of cancer biomarkers using transcription variants. The DYX1C1 gene gives rise to three alternatively spliced transcripts according to the GenBank database. Among them, one is associated with an HERV-H LTR element that could affect the protein-coding region. The other transcripts are not associated with any repeat sequences. Thus, their expression levels were investigated by RT-PCR amplification in various human tissues including matched pairs of normal/tumor tissue samples derived from tumor patients.
Materials and methods
Sample preparation
RNA samples from normal human tissues (adrenal gland, bone marrow, cerebellum, adult brain, fetal brain, fetal liver, heart, kidney, liver, lung, placenta, prostate, salivary gland, skeletal muscle, spinal cord, testis, thymus, thyroid, trachea, and uterus) were purchased from Roche. Matched pairs of adjacent normal/tumor tissues (2 colon, liver, uterus, breast, and stomach) were purchased from Ambion, and normal/tumor frozen tissue samples of colorectal adenoma patients are obtained from the Department of Internal Medicine, College of Medicine Pusan National University. Pure mRNA was extracted using the PolyA Tract mRNA isolation system (Promega).
RT-PCR amplification and sequencing
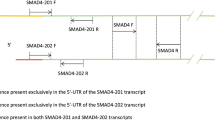
The DYX1C1 gene transcripts were analyzed by RT-PCR amplification. M-MLV reverse transcriptase with an annealing temperature of 42°C was used for reverse transcription reactions with an RNase inhibiter (Promega). The transcript associated with an HERV-H LTR was amplified by the primer pairs S1 (5′-AGG CAC GAA GAG CAA TGA AT-3′) and AS2 (5′-GTG GGG CCG TTT TAT AGG AT-3′), designed using sequence information obtained from GenBank (accession no. NM_001033560). The other two transcripts were amplified by the primer pairs S2 (5′-GAC ATA GCT GAA CTT TGC GAT TT-3′) and AS2 (5′-GAT TTA TAA TAT TTT GCC CTC AAC AG-3′), designed using sequence information from GenBank (accession no. NM_001033559 and NM_130810). For the semi-quantitative RT-PCR analysis, three independent experiments were performed. PCR conditions were as follows: after the initial step of 94°C for 4 min, 30/32/34 cycles of 95°C for 40 s, 55°C for 40 s, and 72°C for 90 s were performed. The primer locations are indicated in Fig. 1. As a standard control, G3PDH was amplified by the primer pairs GPH-S (5′-GAG CCC CAG CCT TCT CCA TG-3′) and GPH-AS (5′-GAA ATC CCA TCA CCA TCT TCC AGG-3′) from human G3PDH (GenBank accession no. NM_002046). These control PCR experiments were also conducted three times using the following conditions: after the initial step of 94°C for 4 min, 30/32/35 cycles of 95°C for 40 s, 55°C for 40 s, and 72°C for 40 s were performed.
Analysis of RT-PCR products
Differentially expressed PCR products were analyzed using the Image J (1.36b) [http://rsb.info.nih.gov/ij/] program to compare the expression levels of each transcript. Image J program can calculate area and pixel value statistics of user-defined selections. The intensity of three alternative transcript including new transcripts were evaluated numerically to compare easily. G3PDH was used as the control.
Results and discussion
Identification and characterization of DYX1C1 alternative transcripts
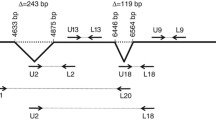
From the GenBank database analysis, three alternatively spliced isoforms of the DYX1C1 gene were identified (Fig. 1). One of the transcripts is associated with an HERV-H LTR element (NM_001033560). The antisense-oriented HERV-H LTR element is located in the last exon of the DYX1C1 gene. An additional coding region and poly-A signal are provided by the HERV-H LTR element. The alternatively spliced transcripts were ubiquitously expressed in several human tissues, and four additional alternative transcripts (V1-1, V1-2, V1-3, and V1-4) were identified using a RT-PCR approach (Fig. 2). The four newly identified transcripts are associated with the HERV-H LTR located in the last exon. New transcripts (V1-1, V1-2, V1-3, and V1-4) acquired the novel exons or lost the original exon from intronic sequences. V1-1 acquired one exon (9′) from intronic sequences compared to V1-2. V1-3 was generated by the deletion of the original eighth exon and the acquisition of a new exon (7′) from intronic sequences. The shortest alternative transcript, V1-4 was generated by excision of the eighth exon due to the selection of alternative splice sites.
One of the most abundant HERV families, HERV-H is distributed throughout the human genome with 100 copies of both the full-length element and solitary LTRs (Sin et al. 2006, 2007). HERV elements have commonly been shown to be responsible for tissue-specific expression by providing promoter or enhancer elements, and other HERVs have appeared to harbor different polyA signals (AATAA) that may alter transcript polyadenylation (Wentzensen et al. 2007). The solitary LTR from HERV-H in DYX1C1 has the potential to regulate its expression pattern by providing a polyA signal (Nelson et al. 1996; de Parseval et al. 1999). NADSYN1 (NAD synthetase) has been shown to have several new alternative transcripts resulting from HERV-H LTR-derived polyA signals and these transcripts are expressed in normal human tissues (Sin et al. 2007).
Application of DYX1C1 alternatively spliced transcripts as cancer biomarkers
HERV-H elements are reported to be strongly expressed in testicular, lung and gastrointestinal cancers (Wentzensen et al. 2007; Wilkinson et al. 1990; Hirose et al. 1993). The accumulation of mutations in HERV sequences that result in cryptic splice sites changes the function of proteins by modifying the expression of transcripts in cancerous tissues. Therefore, we performed semi-quantitative RT-PCR analysis using five normal and adjacent tumor tissue sets (Figs. 3, 4). Among five splice variants (V1, V1-1, V1-2, V1-3 and V1-4), V1 was the predominant transcript in the five normal and tumor tissue samples. However, we could not compare the expression pattern of V1-1 with V1-2 because there is difference in size of only 20 base pairs between them. Therefore, we could not discriminate between the individual transcripts. Intriguingly, RT-PCR analysis of V2 and V3 transcripts show specific expression patterns. Although V3 was generally more strongly expressed than V2 in normal and adjacent tumor tissue sets, their expression levels were different between normal and tumor tissue samples (Fig. 4a, b). Moreover, additional five adjacent normal/tumor colon cancer samples were applied for the confirmation of differential expression pattern. Although, two samples did not show the positive result, three samples showed differential expression pattern in tumor tissues (the line 10 showed weak band of V2 and V3 transcript) (Fig. 4c). For the scarcity of patient adjacent normal/tumor samples, we could not analyze many cases of patient samples. However, V2 and V3 transcript of DYX1C1 could be a good biomarker for colon tumor diagnosis.
Comparative analysis of HERV-H LTR-associated DYX1C1 transcripts (V1, V1-1, V1-2, V1-3, V1-4) in normal and tumor tissue samples. G3PDH indicates the positive control. a Lanes: M size marker, 1 colon (T), 2 colon (N), 3 liver (T), 4 liver (N), 5 uterus (T), 6 uterus (N), 7 breast (T), 8 breast (N), 9 stomach (T), 10 stomach (N). b RT-PCR was conducted three times and G3PDH was used as a control. PCR products were analyzed quantitatively using the Image-J program. The X-axis of the bar graph indicates normal (N)/tumor (T) tissue samples. Lanes: M size marker, 1 colon (T), 2 colon (N), 3 liver (T), 4 liver (N), 5 uterus (T), 6 uterus (N), 7 breast (T), 8 breast (N), 9 stomach (T), 10 stomach (N) and the Y-axis of the bar graph indicates the relative expression levels of the 5 transcripts (V1, V1-1, V1-2, V1-3, and V1-4)
Comparative analysis of the other transcripts (V2, V3) in normal (N) and tumor (T) samples. G3PDH indicates the positive control. a Lanes: M size marker, 1 colon (T), 2 colon (N), 3 liver (T), 4 liver (N), 5 uterus (T), 6 uterus (N), 7 breast (T), 8 breast (N), 9 stomach (T), 10 stomach (N). b RT-PCR was conducted three times and G3PDH was used as a control. PCR products were analyzed quantitatively using the Image-J program. The X-axis of the bar graph indicates normal (N)/tumor (T) tissue samples. Lanes: M size marker, 1 colon (T), 2 colon (N), 3 liver (T), 4 liver (N), 5 uterus (T), 6 uterus (N), 7 breast (T), 8 breast (N), 9 stomach (T), 10 stomach (N) and the Y-axis of the bar graph indicates the relative expression levels of 2 transcripts (V2 and V3). P < 0.05 in Student’s t-test is indicated by *. c Lanes: M size marker, 1 colon (N), 2 colon (T), 3 colon (N), 4 colon (T), 5 colon (N), 6 colon (T), 7 colon (N), 8 colon (T), 9 colon (N), 10 colon (T). Lane 10 showed the weak expression pattern of V2 and V3 transcript. Their expression pattern showed only presence/absence status of transcript, therefore, we did not analyze quantitatively using the Image-J program
The expression levels of DYX1C1 transcripts seem to be altered by the progression of tumors specifically in colorectal tissue. The DYX1C1 transcript could be useful as a molecular marker to detect colorectal cancer (Fig. 4). A typical model of splice variant-derived variations in expression patterns between normal and tumor tissues is found with the Survivin gene. It is strongly expressed in cancer tissues. However, it could not be detected in normal tissues, indicating a potential role in tumorigenesis (Li 2005). Alternative splice variants of survivin show different expression patterns during tumor progression (Pajares et al. 2007; Li 2005; Mahotka et al. 1999; Badran et al. 2004; Krieg et al. 2002; Meng et al. 2004).
Taken together, alternative transcripts of DYX1C1 could be applied as cancer biomarkers to detect colorectal cancer. The different proteins derived from the V2 and V3 transcripts need to be analyzed further in studies using various colorectal normal and tumor tissue samples.
Conclusion
Our results indicated that alternatively spliced transcript variants of the DYX1C1 gene could be used as cancer biomarkers to detect colorectal cancer using a quantitative expression approach.
References
Badran A, Yoshida A, Ishikawa K, Goi T, Yamaguchi A, Ueda T et al (2004) Identification of a novel splice variant of the human anti-apoptopsis gene survivin. Biochem Biophys Res Commun 314:902–907. doi:10.1016/j.bbrc.2003.12.178
Brinkman BM (2004) Splice variants as cancer biomarkers. Clin Biochem 37:584–594. doi:10.1016/j.clinbiochem.2004.05.015
Chang BS, Ly J, Appignani B, Bodell A, Apse KA, Ravenscroft RS et al (2005) Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology 64:799–803
de Parseval N, Alkabbani H, Heidmann T (1999) The long terminal repeats of the HERV-H human endogenous retrovirus contain binding sites for transcriptional regulation by the Myb protein. J Gen Virol 80:841–845
Fisher SE, Francks C (2006) Genes, cognition and dyslexia: learning to read the genome. Trends Cogn Sci 10:250–257. doi:10.1016/j.tics.2006.04.003
Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N (1985) Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol 18:222–233. doi:10.1002/ana.410180210
Ge K, DuHadaway J, Du W, Herlyn M, Rodeck U, Prendergast GC (1999) Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc Natl Acad Sci USA 96:9689–9694. doi:10.1073/pnas.96.17.9689
Hirose Y, Takamatsu M, Harada F (1993) Presence of env genes in members of the RTVL-H family of human endogenous retrovirus-like elements. Virology 192:52–61. doi:10.1006/viro.1993.1007
Krieg A, Mahotka C, Krieg T, Grabsch H, Müller W, Takeno S et al (2002) Expression of different survivin variants in gastric carcinomas: first clues to a role of survivin–2B in tumour progression. Br J Cancer 86:737–743. doi:10.1038/sj.bjc.6600153
Li F (2005) Role of survivin and its splice variants in tumorigenesis. Br J Cancer 92:212–216
Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD (1999) Survivin-deltaEx3 and survivin–2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res 59:6097–6102
McGrath LM, Smith SD, Pennington BF (2006) Breakthroughs in the search for dyslexia candidate genes. Trends Mol Med 12:333–341. doi:10.1016/j.molmed.2006.05.007
Meng H, Lu CD, Sun YL, Dai DJ, Lee SW, Tanigawa N (2004) Expression level of wild-type survivin in gastric cancer is an independent predictor of survival. World J Gastroenterol 10:3245–3250
Nelson DT, Goodchild NL, Mager DL (1996) Gain of Sp1 sites and loss of repressor sequences associated with a young, transcriptionally active subset of HERV-H endogenous long terminal repeats. Virology 220:213–218. doi:10.1006/viro.1996.0303
Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, Montuenga LM (2007) Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol 8:349–357. doi:10.1016/S1470-2045(07)70104-3
Pennington BF, Smith SD (1983) Genetic influences on learning disabilities and speech and language disorders. Child Dev 54:369–387. doi:10.2307/1129698
Ryan B, O’Donovan N, Browne B, O’Shea C, Crown J, Hill AD et al (2005) Expression of survivin and its splice variants survivin-2B and survivin-DeltaEx3 in breast cancer. Br J Cancer 92:120–124. doi:10.1038/sj.bjc.6602314
Sin HS, Huh JW, Kim DS, Kim TH, Ha HS, Kim WY et al (2006) Endogenous retrovirus-related sequences provide an alternative transcript of MCJ genes in human tissues and cancer cells. Genes Genet Syst 1:333–339. doi:10.1266/ggs.81.333
Sin HS, Huh JW, Kim WY, Kim DS, Ahn K, Ha HS et al (2007) Long terminal repeats of human endogenous retrovirus H family provide alternative polyadenylation signals to NADSYN1 gene. Korean J Genet 29:395–401
Skotheim RI, Nees M (2007) Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol 39:1432–1449. doi:10.1016/j.biocel.2007.02.016
Smith SD, Kelley PM, Brower AM (1998) Molecular approaches to the genetic analysis of specific reading disability. Hum Biol 70:239–256
Sokol DK, Golomb MR, Carvalho KS, Edwards-Brown M (2006) Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology 66:294 (author reply 294)
Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, Lyytinen H et al (2003) A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc Natl Acad Sci USA 100:11553–11558. doi:10.1073/pnas.1833911100
Venables JP (2004) Aberrant and alternative splicing in cancer. Cancer Res 64:7647–7654. doi:10.1158/0008-5472.CAN-04-1910
Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, Kere J et al (2006) DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience 143:515–522. doi:10.1016/j.neuroscience.2006.08.022
Wilkinson DA, Freeman JD, Goodchild NL, Kelleher CA, Mager DL (1990) Autonomous expression of RTVL-H endogenous retroviruslike elements in human cells. J Virol 64:2157–2167
Wentzensen N, Coy JF, Knaebel HP, Linnebacher M, Wilz B, Gebert J et al (2007) Expression of an endogenous retroviral sequence from the HERV-H group in gastrointestinal cancers. Int J Cancer 121:1417–1423. doi:10.1002/ijc.22826
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (0620150-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yun-Ji Kim and Jae-Won Huh contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, YJ., Huh, JW., Kim, DS. et al. Molecular characterization of the DYX1C1 gene and its application as a cancer biomarker. J Cancer Res Clin Oncol 135, 265–270 (2009). https://doi.org/10.1007/s00432-008-0445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0445-8