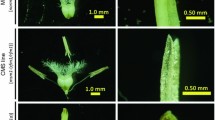
Abstract
The rice lesion mimic mutant spotted leaf 1 ( spl1) was first identified in the rice ( Oryza sativa) cultivar Asahi in 1965. This mutant displayed spontaneous disease-like lesions in the absence of any pathogen, and was found to confer resistance to multiple isolates of rice blast. We employed a map-based cloning strategy to localize the Spl1 gene. A total of ten cleaved amplified polymorphic sequence (CAPS) markers linked to the Spl1 gene were identified and mapped to an 8.5-cM region on chromosome 12. A high-resolution genetic map was developed using these ten CAPS markers and a segregating population consisting of 3202 individuals. A BAC contig containing four BAC clones was constructed, and Spl1 was localized to a 423-kb region. Seven spl1 mutants were obtained from the IR64 deletion mutant collection, and molecular analysis using these mutants delimited the Spl1 gene to a 70-kb interval, covered by two BAC clones. These results provide the basis for cloning this gene, which is involved in cell death and disease resistance in rice.




Similar content being viewed by others
References
Arase S, Fujita K, Uehara T, Honda Y, Isota J (2000) Light-enhanced resistance to Magnaporthe grisea infection in the rice Sekiguchi lesion mutants. J Phytopathol 148:197–203
Arase S, Ueno M, Toko M, Honda Y, Itho K, Ozoe Y (2001) Light-dependent accumulation of tryptamine in the rice Sekiguchi lesion mutant infected with Magnaporthe grisea. J Phytopathol 149:409–413
Bent AF (1996) Plant disease resistance genes: function meets structure. Plant Cell 8:1757–1771
Bradeen JM, Simon PW (1998) Conversion of an AFLP fragment linked to the carrot Y2 locus to a simple, co-dominant, PCR-based marker form. Theor Appl Genet 97:960–967
Bruggemann E, Handwerger K, Essex C, Storz G (1996) Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J 10:755-760
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, Van Daelen R, Van der Lee T, Dergaarde P, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705
Chen M-S, et al (2002) An integrated physical and genetic map of the rice genome. Plant Cell 14:537–545
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8:1793–1807
Dellaporta SL, Wood J, Hicks JB (1984) Maize DNA miniprep. In: Russell M (ed) Molecular biology of plants: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 36–37
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88:685–694
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Llsl gene of maize. Cell 89:25–31
Greenberg JT (1997) Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol 48:525–545
Greenberg JT, Ausubel FM (1993) Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 4:327–341
Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8:1773–1791
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Hu G, Yalpani N, Briggs SP, Johal GS (1998) A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 10:1095–1105
Isota J, Kadowaki Y, Arase S (1996) Spontaneous occurrence of the Sekiguchi lesion on rice cv. Himenomochi. Ann Phytopathol Soc Jpn 62:167–169
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273:1853–1856
Kinoshita CT (1995) Report of committee on gene symbolization, nomenclature and linkage groups. Rice Genet Newslett 12:9–115
Kiyosawa S (1970) Inheritance of a particular sensitivity of the rice variety, Sekiguchi Asahi, to pathogens and chemicals, and linkage relationship with blast resistance. Bull Nat Inst Agric Sci (Jpn) Ser D Physiol Genet 21:61–71
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848-853
Leung H, et al (2001) Deletion mutants for functional genomics: progress in phenotyping, sequence assignment, and database development. In: Rice Genetics IV. Science Publishers, New Delhi, pp 239–251
Lorrain S, Vailleau F, Balague C, Roby D (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8:263–271
Marchetti MA, Bollich CN, Uecker FA (1983) Spontaneous occurrence of the Sekiguchi lesion in two American rice lines: its induction, inheritance, and utilization. Phytopathology 73:603–606
Mizobuchi R, Hirabayashi H, Kaji R, Nishizawa Y, Yoshimura A, Satoh H, Ogawa T, Okamoto M (2002) Isolation and characterization of rice lesion-mimic mutants with enhanced resistance to rice blast and bacterial blight. Plant Sci 163:345–353
Nakamura S, Asakawa S, Ohmido N, Fukui K, Shimizu N, Kawasaki S (1997) Construction of an 800-kb contig in the near-centromeric region of the rice blast resistance gene Pi-ta 2 using a highly representative rice BAC library. Mol Gen Genet 254:611–620
Okubara PA, Anderson PA, Ochoa OE, Michelmore RW (1994) Mutants of downy mildew resistance in Lactuca sativa (lettuce). Genetics 137:867-874
Reardon JT, Liljestrand-Golden CA, Dusenbery RL, Smith PD (1987) Molecular analysis of diepoxybutane-induced mutations at the rosy locus of Drosophila melanogaster. Genetics 115:323-331
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor Laboratory, N.Y.
Sekiguchi Y, Furuta T (1965) On a rice mutant showing particular reaction to some spotting disease. Preliminary report. Ann Phytopathol Soc Jpn 30:71–72 (in Japanese)
Sherman JD, Stack SM (1995) Two-dimensional spreads of synaptonemal complexes from solanaceous plants. High-resolution recombination nodule map for tomato ( Lycopersicon esculentum). Genetics 141:683–708
Shirley BS, Hanley S, Goodman HM (1992) Effects of ionizing radiation on a plant genome: analysis of two Arabidopsis transparent testa mutations. Plant Cell 4:333-347
Simmons C, Hantke S, Grant S, Johal GS, Briggs SP (1998) The maize lethal leaf spot 1 mutant has elevated resistance to fungal infection at the leaf epidermis. Mol Plant-Microbe Interact 11:1110–1118
Singh K, Multani DS, Khush GS (1995) A new spotted leaf mutant in rice. Rice Genet Newslett 12:192–193
Tanksley SD, et al (1992) High density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Tarchini R, Biddle P, Wineland R, Tingey S, Rafalski A (2000) The complete sequence of 340 kb of DNA around the rice Adh1 - Adh2 region reveals interrupted colinearity with maize chromosome 4. Plant Cell 12:381-391
Tomiyama K (1963) Physiology and biochemistry of disease resistance of plants. Annu Rev Phytopathol 1:295–324
Ueno M, Shibata H, Kihara J, Honda Y, Arase S (2003) Increased tryptophan decarboxylase and monoamine oxidase activities induce Sekiguchi lesion formation in rice infected with Magnaporthe grisea. Plant J 36:215–228
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19:55-64
Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K (2002) A rice spotted leaf gene, Spl7 , encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA 99:7530–7535
Yin ZC, Chen J, Zeng LR, Goh M, Leung H, Khush GS, Wang GL (2000) Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol Plant-Microbe Interact 13:869–876
Zeng L, Yin Z, Chen J, Leung H, Wang GL (2002) Fine genetic mapping and physical delimitation of the lesion mimic gene Spl11 to a 160 kb DNA segment of the rice genome. Mol Genet Genomics 268:253–261
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30028016) and the Basic Research Program (The ‘973’ Program, Grant No. TG2000016203) of the Ministry of Science and Technology of China. Work at IRRI was supported in part by the Swiss Agency for Cooperation and Development. We thank Alice Bordeos for assistance in genetic analysis and Bill Hardy for technical editing
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
The first two authors contributed equally to the work
Rights and permissions
About this article
Cite this article
Liu, G., Wang, L., Zhou, Z. et al. Physical mapping of a rice lesion mimic gene, Spl1 , to a 70-kb segment of rice chromosome 12. Mol Genet Genomics 272, 108–115 (2004). https://doi.org/10.1007/s00438-004-1040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-1040-6