Abstract
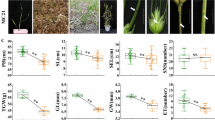
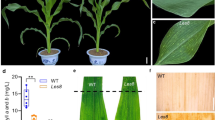
Lesion mimic mutants are characterized by the formation of necrotic lesions in the absence of pathogens. Such genetic defects often result in enhanced resistance to pathogen infection and constitutive expression of defense response genes. To understand the genetic mechanisms leading to these mutations, we characterized 21 lesion mimic mutants isolated from IR64 rice mutant populations produced by mutagenesis with diepoxybutane (D), gamma rays (G), and fast neutrons (F). Four mutations are controlled by single dominant genes, one of which is inherited maternally. Five lesion mimics are allelic to known spotted leaf (spl) mutants spl1, spl2, spl3, or spl6. In total, 11 new lesion mimic mutations, named spl16, spl17, and spl19 through Spl27, were established based on allelism tests. Two lesion mimics, spl17 and Spl26 showed enhanced resistance to multiple strains of Magnaporthe oryzae, the rice blast pathogen, and Xanthomonas oryzae pv. oryzae, the bacterial blight (BB) pathogen. Co-segregation analyses of blast and BB resistance and lesion mimic phenotypes in segregating populations of spl17 and Spl26 indicate that enhanced resistance to the two diseases is conferred by mutations in the lesion mimic genes. A double mutant produced from two independent lesion mimics showed more severe lesions and higher level of resistance to X. o. pv. oryzae than their single mutant parents indicating a synergistic effect of the two mutations. In mutants that exhibit enhanced disease resistance to both pathogens, increases in expression of defense response genes PR-10a, POX22.3, and PO-C1 were correlated with lesion mimic development and enhancement of resistance. These lesion mimic mutants may provide essential materials for a comprehensive dissection of the disease resistance pathways in rice.







Similar content being viewed by others
References
Arase S, Zhao C-M, Akimitsu K, Yamamoto M, Ichii M (2000) A recessive lesion mimic mutant of rice with elevated resistance to fungal pathogens. J Gen Plant Pathol 66:109–116
Balague C, Lin B, Alcon C, Flottes G, Malmstrom S, Kohler C, Neuhaus G, Pelletier G, Gaymard F, Roby D (2003) HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15:365–379
Bonman JM, Vergel de Dios TI, Khin MM (1986) Physiologic specialization of Pyricularia oryzae in the Philippines. Plant Dis 70:767–769
Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Odum N, Jorgensen LB, Brown RE, Mundy J (2002) Knockout of Arabidopsis accelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev 16:490–502
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705
Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6:1583–1592
Chitoor JM, Leach JE, White FF (1997) Differential induction of a peroxidase gene family during infection of rice by Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 10:861–871
Cooper B, Clarke JD, Budworth P, Kreps J, Hutchison D, Park S, Guimil S, Dunn M, Luginbühl P, Ellero C, Goff SA, Glazebrook J (2003) A network of rice genes associated with stress response and seed development. PNAS 100:4945–4950
Couch BC, Kohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94:683–693
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc-finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88:685–694
Fuse T, Iba K, Satoh H, Nishimura M (1993) Characterization of a rice mutant having an increased susceptibility to light stress at high temperature. Physiol Plant 89:799–804
Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143:973–982
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89:25–31
Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS (2002) Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol 130:1894–1907
Greenberg JT, Guo A, Klessig DF, Ausubel FM (1994) Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 77:551–563
Guo A, Reimers PJ, Leach JE (1993) Effect of light on incompatible interactions between Xanthomonas oryzae pv. oryzae and rice. Physiol Mol Plant Pathol 42:413–425
Hilaire E, Young SA, Willard LH, McGee JD, Sweat T, Chittoor JM, Guikema JA, Leach JE (2001) Vascular defense responses in rice: peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol Plant Microbe Interact 14:1411–1419
Hoisington DA, Neuffer MG, Walbot V (1982) Disease lesion mimics in maize I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1. Dev Biol 93:381–388
Hu G, Richter TE, Hulbert SH, Pryor T (1996) Disease lesion mimicry caused by mutations at the rust resistance gene rp1. Plant Cell 8:1367–1376
Hu G, Yalpani N, Briggs SP, Johal GS (1998) A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 10:1095–1106
Ideta O, Yoshimura A, Matsumoto T, Tsunematsu H, Saito H, Iwata N (1993) Integration of conventional and RFLP linkage maps in rice, II. Chromosome 6, 9, 10 and 11 Rice Genet News 10:87–89
Ishikawa A, Okamoto H, Iwasaki Y, Asahi T (2001) A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. Plant J 27:89–99
Iwata N, Omura T, Satoh H (1978) Linkage studies in rice (Oryza sativa L.). On some mutants for physiological leaf spots. J Fac Agr Kyushu Univ 22:243–251
Johal G (2007) Disease lesion mimic mutants of maize. APSnet July 2007. http://www.apsnet.org/online/feature/mimics/default.asp. Cited 24 January 2008
Johal GS, Hulbert S, Briggs SP (1995) Disease lesion mimics of maize: a model for cell death in plants. Bioessays 17:685–692
Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA 98:9448–9453
Kauffman HE, Reddy APK, Hsieh SPY, Merca SD (1973) An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep 57:537–541
Leung H, Wu C, Baraoidan M, Bordeos A, Ramos M, Madamba S, Cabauatan P, Vera Cruz C, Portugal A, Reyes G, Bruskiewich R, McLaren G, Lafitte G, Gregorio G, Bennett J, Brar D, Khush G, Schnable P, Wang GL, Leach JE (2001) Deletion mutants for functional genomics: progress in phenotyping, sequence assignment, and database development. In: Khush GS, Brar DS, Hardy B (eds) Rice genetics iv (proceedings of the fourth international rice genetics symposium, 22–27 October 2000, Los Baños, Philippines) Science Publishers Inc, New Delhi, India and International Rice Research Institute, Los Baños, Philippines, pp 239–251
Lorrain S, Lin B, Auriac MC, Kroj T, Saindrenan P, Nicole M, Balagué C, Roby D (2004) Vascular associated death1, a novel GRAM domain-containing protein, is a regulator of cell death and defense responses in vascular tissues. Plant Cell 16:2217–32
Lorrain S, Vailleau F, Balague C, Roby D (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8:263–271
Lyngkjaer MF, Newton AC, Atzema JL, Baker SJ (2000) The Barley mlo-gene: an important powdery mildew resistance source. Agronomie 20:745–756
Marchetti MA, Bollich CN, Uecker FA (1983) Spontaneous occurrences of the Sekiguchi lesion in two American rice lines: its induction, inheritance and utilization. Phytopathol 73:603–606
Martienssen R, Irish V (1999) Copying out our ABCs: the role of gene redundancy in interpreting genetic hierarchies. Trends Genet 15:435–437
McGee JD, Hamer JE, Hodges TK (2001) Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant Microbe Interact 14:877–886
Mizobuchi R, Hirabayashi H, Kaji R, Nishizawa Y, Yoshimura A, Satoh H, Ogawa T, Okamoto M (2002a) Isolation and characterization of rice lesion-mimic mutants with enhanced resistance to rice blast and bacterial blight. Plant Sci 163:345–353
Mizobuchi R, Hirabayashi H, Kaji R, Nishizawa Y, Satoh H, Ogawa T, Okamoto M (2002b) Differential expression of disease resistance in rice lesion-mimic mutants. Plant Cell Rep 21:390–396
Mori M, Tomita C, Sugimoto K, Hasegawa M, Hayashi N, Dubouzet JG, Ochiai H, Sekimoto H, Hirochika H, Kikuchi S (2007) Isolation and molecular characterization of a Spotted leaf 18 mutant by modified activation-tagging in rice. Plant Mol Biol 63:847–860
Neuffer MG, Calvert OH (1975) Dominant disease lesion mimics in maize. J Hered 66:265–270
Pryor AJ (1987) The origin and structure of fungal disease resistance genes in plants. Trends Genet 3:157–161
Reimers PJ, Guo A, Leach JE (1992) Increased activity of a cationic peroxidase associated with an incompatible interaction between Xanthomonas oryzae pv oryzae and rice (Oryza sativa). Plant Physiol 99:1044–1050
Roumen EC, Bonman JM, Parleviet JE (1992) Leaf age related partial resistance to Pyricularia oryzae in tropical lowland rice cultivars as measured by the number of sporulating lesions. Phytopathology 82:1414–1417
Rusterucci C, Aviv DH, Holt BF, Dangl JL, Parker JE (2001) The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13:2211–2224
Shah J, Kachroo P, Klessig DF (1999) The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 11:191–206
Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid–insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10:69–78
Shirano Y, Kachroo P, Shah J, Klessig DF (2002) A gain-of function mutation in an Arabidopsis toll interleukin1 receptor nucleotide binding site-leucine-rich repeat type R gene triggers defence responses and results in enhanced disease resistance. Plant Cell 14:3149–3162
Singh K, Multani DS, Khush GS (1995) A new spotted leaf mutant in rice. Rice Genet Newsl 12:192–193
Takahashi A, Kawasaki T, Henmi K, Shii K, Kodama O, Satoh H, Shimamoto K (1999) Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J 17:535–545
Takahashi M, Kinoshita T, Takeda K (1968) Character expression and causal genes of some mutants in rice plant (Genetical studies on rice plant, XXXIII). J Fac Agr Hokkaido Univ 55:496–512
Walbot V (1991) Maize mutants for the 21st century. Plant Cell 3:851–856
Wu J, Wu C, Lei C, Baraoidan M, Bordeos A, Madamba RS, Ramos-Pamplona M, Mauleon R, Portugal A, Ulat V, Bruskiewich R, Wang GL, Leach JE, Khush G, Leung H (2005) Chemical- and irradiation-induced mutants of indica rice IR64 for forward and reverse genetics. Plant Mol Biol 59:85–97
Yamamoto T, Hifni HR, Machmud M, Nizhikawa T, Tantera DM (1977) Variation in pathogenicity of Xanthomonas oryzae (Uyeda et al. Ishiyama) Dowson and resistance of rice varieties to the pathogen. Contr Gen Agric Res Stn, Bogor, Indonesia no 8, 22 pp
Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K (2002) A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA 99:7530–7535
Yin Z, Chen J, Zeng L, Goh M, Leung H, Khush GS, Wang GL (2000) Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol Plant Microbe Interact 13:869–876
Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm BH, Leung H, Wang GL (2004) Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-Box/Armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 16:2795–2808
Acknowledgments
We thank the technical help of Pedro Reaño, Alexander Ramos, and Benedicto Consignado. We also thank Violeta Bartolome for assistance in statistical analyses. The work was supported in part by grants from the Rockefeller Foundation and Swiss Development Cooperation (HL) and USDA-CSREES-NRI grant 2003–3519 13285 (JEL and HL), the Colorado Agricultural Experiment Station (JEL).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Wu and A. Bordeos contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
438_2008_337_MOESM2_ESM.ppt
Figure S1. Differences in reduction in lesion lengths in IR64-derived lesion mimic spl17 relative to IR64 when inoculated with four Philippine strains of X. oryzae pv. oryzae in two tests (done in October 2004 and February 2005). Reduction in lesion length was greater in the October 2004 test. Effect of different environmental conditions (temperature and solar radiation) prevailing during the growth period of the plants on degree of mimic expression is inferred. Data on daily solar radiation and temperature were taken at the IRRI Experimental Station and provided by the IRRI Climate Unit. Error bars in the lesion lengths indicate + SE from averages of three replications. (PPT 208 kb)
Rights and permissions
About this article
Cite this article
Wu, C., Bordeos, A., Madamba, M.R.S. et al. Rice lesion mimic mutants with enhanced resistance to diseases. Mol Genet Genomics 279, 605–619 (2008). https://doi.org/10.1007/s00438-008-0337-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-008-0337-2