Abstract
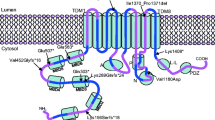
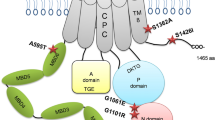
The genes for two copper-transporting ATPases, ATP7A and ATP7B, are defective in the heritable disorders of copper imbalance, Menkes disease (MNK) and Wilson disease (WND), respectively. A comparison of the two proteins shows extensive conservation in the signature domains, with amino acid identities outside of the conserved domains being limited. The mutation spectra of MNK and WND were compared to confirm and refine further regions critical for normal function. Mutations were found to be relatively widespread; however, the majority was concentrated within defined functional domains and membrane-spanning segments, reinforcing the importance of these regions for protein function. Of the total published point mutations in ATP7A, 23.0% are splice-site, 20.7% nonsense, 17.2% missense, and 39.1% small insertions/deletions. There is a high prevalence (58.2%) of missense mutations in ATP7B. For the other mutations in ATP7B, 7.4% are splice-site, 7.4% nonsense, and 27.0% small insertions/deletions. A region of possible importance is the intervening sequence between the last copper-binding domain and the first transmembrane helix, as this region has a high percentage of MNK mutations. Similarly, the region containing the ATP-binding domain has 24.6% of all WND mutations. The study of mutation locations is useful for defining critical regions or residues and for efficient molecular diagnosis.

Similar content being viewed by others
References
Ambrosini L, Mercer JF (1999) Defective copper-induced trafficking and localization of the Menkes protein in patients with mild and copper-treated classical Menkes disease. Hum Mol Genet 8:1547–1555
Beck J, Enders H, Schliephacke M, Buchwald-Saal M, Tümer Z (1994) X;1 translocation in a female Menkes patient: characterization by fluorescence in situ hybridization. Clin Genet 46:295–298
Bissig KD, Wunderli-Ye H, Duda PW, Solioz M (2001) Structure-function analysis of purified Enterococcus hirae CopB copper ATPase: effect of Menkes/Wilson disease mutation homologues. Biochem J 357:217–223
Bull PC, Cox DW (1994) Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet 10:246–252
Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5:327–337
Byers PH, Siegel RC, Holbrook KA, Narayanan AS, Bornstein P, Hall JG (1980) X-linked cutis laxa: defective cross-link formation in collagen due to decreased lysyl oxidase activity. N Engl J Med 303:61–65
Camakaris J, Voskoboinik I, Mercer JF (1999) Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun 261:225–232
Chelly J, Tümer Z, Tönnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP (1993) Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 3:14–19
Cox, Roberts EA (2002) Wilson disease. In: Feldman M, Friedman LS, Sleisenger MH (eds) Sleisenger and Forstran’s gastrointestinal and liver disease: pathophysiology/diagnosis/management, 7th edn. Saunders, Philadelphia, pp 1269–1277
Dagenais SL, Adam AN, Innis JW, Glover TW (2001) A novel frameshift mutation in exon 23 of ATP7A (MNK) results in occipital horn syndrome and not in Menkes disease. Am J Hum Genet 69:420–427
Danks (1995) Disorders of copper transport. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited disease, 7th edn. McGraw-Hill, New York, pp 2211–2235
Das S, Levinson B, Whitney S, Vulpe C, Packman S, Gitschier J (1994) Diverse mutations in patients with Menkes disease often lead to exon skipping. Am J Hum Genet 55:883–889
Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S (1995) Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet 56:570–576
DiDonato M, Sarkar B (1997) Copper transport and its alterations in Menkes and Wilson diseases. Biochim Biophys Acta 1360:3–16
Fatemi N, Sarkar B (2002) Structural and functional insights of Wilson disease copper-transporting ATPase. J Bioenerg Biomembr 34:339–349
Forbes JR, Cox DW (1998) Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am J Hum Genet 63:1663–1674
Forbes JR, Cox DW (2000) Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum Mol Genet 9:1927–1935
Forbes JR, Hsi G, Cox DW (1999) Role of the copper-binding domain in the copper transport function of ATP7B, the P-type ATPase defective in Wilson disease. J Biol Chem 274:12408–12413
Gu YH, Kodama H, Murata Y, Mochizuki D, Yanagawa Y, Ushijima H, Shiba T, Lee CC (2001) ATP7A gene mutations in 16 patients with Menkes disease and a patient with occipital horn syndrome. Am J Med Genet 99:217–222
Hahn S, Cho K, Ryu K, Kim J, Pai K, Kim M, Park H, Yoo O (2001) Identification of four novel mutations in classical Menkes disease and successful prenatal DNA diagnosis. Mol Genet Metab 73:86–90
Kaler SG (1998) Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatr Dev Pathol 1:85–98
Kaler SG, Gallo LK, Proud VK, Percy AK, Mark Y, Segal NA, Goldstein DS, Holmes CS, Gahl WA (1994) Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet 8:195–202
Kaler SG, Buist NR, Holmes CS, Goldstein DS, Miller RC, Gahl WA (1995) Early copper therapy in classic Menkes disease patients with a novel splicing mutation. Ann Neurol 38:921–928
Kapur S, Higgins JV, Delp K, Rogers B (1987) Menkes syndrome in a girl with X-autosome translocation. Am J Med Genet 26:503–510
La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Parton RG, Camakaris J, Mercer JF (1998) Functional analysis and intracellular localization of the human menkes protein (MNK) stably expressed from a cDNA construct in Chinese hamster ovary cells (CHO-K1). Hum Mol Genet 7:1293–1300
Levinson B, Conant R, Schnur R, Das S, Packman S, Gitschier J (1996) A repeated element in the regulatory region of the MNK gene and its deletion in a patient with occipital horn syndrome. Hum Mol Genet 5:1737–1742
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S-811S
Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM (2002) Human copper-transporting ATPase ATP7B (the Wilson’s disease protein): biochemical properties and regulation. J Bioenerg Biomembr 34:351–362
Mak BS, Chi CS, Tsai CR (2002) Menkes gene study in the Chinese population. J Child Neurol 17:250–252
Menkes JH, Alter M, Steigleder G, Weakley DR, Sun JH (1962) A sex-linked recessive disorder with retardation of growth, peculiar hair and focal cerebral and cerebellar degeneration. Pediatrics 29:764–779
Møller LB, Tümer Z, Lund C, Petersen C, Cole T, Hanusch R, Seidel J, Jensen LR, Horn N (2000) Similar splice-site mutations of the ATP7A gene lead to different phenotypes: classical Menkes disease or occipital horn syndrome. Am J Hum Genet 66:1211–1220
Ogawa A, Yamamoto S, Takayanagi M, Kogo T, Kanazawa M, Kohno Y (1999a) An Ile/Val polymorphism at codon 1464 of the ATP7A gene. J Hum Genet 44:423–424
Ogawa A, Yamamoto S, Takayanagi M, Kogo T, Kanazawa M, Kohno Y (1999b) Identification of three novel mutations in the MNK gene in three unrelated Japanese patients with classical Menkes disease. J Hum Genet 44:206–209
Ogawa A, Yamamoto S, Kanazawa M, Ogawa E, Takayanagi M, Hasegawa S, Kohno Y (2000) Novel mutation of L718X in the ATP7A gene in a Japanese patient with classical Menkes disease, and four novel polymorphisms in the Japanese population. J Hum Genet 45:315–317
Ozawa H, Kodama H, Murata Y, Takashima S, Noma S (2001) Transient temporal lobe changes and a novel mutation in a patient with Menkes disease. Pediatr Int 43:437–440
Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J 15:6084–6095
Petris MJ, Camakaris J, Greenough M, LaFontaine S, Mercer JF (1998) A C-terminal di-leucine is required for localization of the Menkes protein in the trans-Golgi network. Hum Mol Genet 7:2063–2071
Poulsen L, Horn N, Heilstrup H, Lund C, Tümer Z, Møller LB (2002) X-linked recessive Menkes disease: identification of partial gene deletions in affected males. Clin Genet 62:449–457
Proud VK, Mussell HG, Kaler SG, Young DW, Percy AK (1996) Distinctive Menkes disease variant with occipital horns: delineation of natural history and clinical phenotype. Am J Med Genet 65:44–51
Qi M, Byers PH (1998) Constitutive skipping of alternatively spliced exon 10 in the ATP7A gene abolishes Golgi localization of the menkes protein and produces the occipital horn syndrome. Hum Mol Genet 7:465–469
Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119:782–793
Ronce N, Moizard MP, Robb L, Toutain A, Villard L, Moraine C (1997) A C2055T transition in exon 8 of the ATP7A gene is associated with exon skipping in an occipital horn syndrome family. Am J Hum Genet 61:233–238
Schaefer M, Hopkins RG, Failla ML, Gitlin JD (1999) Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol 276:G639-G646
Solioz M, Vulpe C (1996) CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci 21:237–241
Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405:647–655
Tümer Z, Horn N (1997) Menkes disease: recent advances and new aspects. J Med Genet 34:265–274
Tümer Z, Tommerup N, Tönnesen T, Kreuder J, Craig IW, Horn N (1992) Mapping of the Menkes locus to Xq13.3 distal to the X-inactivation center by an intrachromosomal insertion of the segment Xq13.3-q21.2. Hum Genet 88:668–672
Tümer Z, Tönnesen T, Bohmann J, Marg W, Horn N (1994a) First trimester prenatal diagnosis of Menkes disease by DNA analysis. J Med Genet 31:615–617
Tümer Z, Tönnesen T, Horn N (1994b) Detection of genetic defects in Menkes disease by direct mutation analysis and its implications in carrier diagnosis. J Inherit Metab Dis 17:267–270
Tümer Z, Vural B, Tönnesen T, Chelly J, Monaco AP, Horn N (1995) Characterization of the exon structure of the Menkes disease gene using vectorette PCR. Genomics 26:437–442
Tümer Z, Horn N, Tönnesen T, Christodoulou J, Clarke JT, Sarkar B (1996) Early copper-histidine treatment for Menkes disease. Nat Genet 12:11–13
Tümer Z, Lund C, Tolshave J, Vural B, Tönnesen T, Horn N (1997) Identification of point mutations in 41 unrelated patients affected with Menkes disease. Am J Hum Genet 60:63–71
Tümer Z, Møller LB, Horn N (1999) Mutation spectrum of ATP7A, the gene defective in Menkes disease. Adv Exp Med Biol 448:83–95
Voskoboinik I, Greenough M, La Fontaine S, Mercer JF, Camakaris J (2001) Functional studies on the Wilson copper P-type ATPase and toxic milk mouse mutant. Biochem Biophys ResCommun 281:966–970
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3:7–13
Acknowledgements
This study was funded by a grant from the Canadian Institutes of Health Research (CIHR) to D.W.C., and G.H. was supported by postgraduate scholarships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Alberta Heritage Foundation for Medical Research (AHFMR).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Hsi, G., Cox, D.W. A comparison of the mutation spectra of Menkes disease and Wilson disease. Hum Genet 114, 165–172 (2004). https://doi.org/10.1007/s00439-003-1045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-003-1045-y