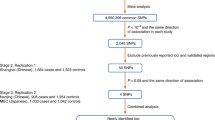
Abstract
Multiple prostate cancer (PCa) risk-related loci have been discovered by genome-wide association studies (GWAS) based on case–control designs. However, GWAS findings may be confounded by population stratification if cases and controls are inadvertently drawn from different genetic backgrounds. In addition, since these loci were identified in cases with predominantly sporadic disease, little is known about their relationships with hereditary prostate cancer (HPC). The association between seventeen reported PCa susceptibility loci was evaluated with a family-based association test using 1,979 hereditary PCa families of European descent collected by members of the International Consortium for Prostate Cancer Genetics, with a total of 5,730 affected men. The risk alleles for 8 of the 17 loci were significantly over-transmitted from parents to affected offspring, including SNPs residing in 8q24 (regions 1, 2 and 3), 10q11, 11q13, 17q12 (region 1), 17q24 and Xp11. In subgroup analyses, three loci, at 8q24 (regions 1 and 2) plus 17q12, were significantly over-transmitted in hereditary PCa families with five or more affected members, while loci at 3p12, 8q24 (region 2), 11q13, 17q12 (region 1), 17q24 and Xp11 were significantly over-transmitted in HPC families with an average age of diagnosis at 65 years or less. Our results indicate that at least a subset of PCa risk-related loci identified by case–control GWAS are also associated with disease risk in HPC families.
Similar content being viewed by others
References
Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le Roux L, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Balter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K (2006) A common variant associated with prostate cancer in European and African populations. Nat Genet 38:652–658. doi:10.1038/ng1808
Duggan D, Zheng SL, Knowlton M, Benitez D, Dimitrov L, Wiklund F, Robbins C, Isaacs SD, Cheng Y, Li G, Sun J, Chang BL, Marovich L, Wiley KE, Balter K, Stattin P, Adami HO, Gielzak M, Yan G, Sauvageot J, Liu W, Kim JW, Bleecker ER, Meyers DA, Trock BJ, Partin AW, Walsh PC, Isaacs WB, Gronberg H, Xu J, Carpten JD (2007) Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst 99:1836–1844. doi:10.1093/jnci/djm250
Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF (2008) Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 40:316–321. doi:10.1038/ng.90
Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, Albers-Akkers MT, Godino-Ivan Marcos J, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2007a) Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39:631–637. doi:10.1038/ng1999
Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2007b) Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39:977–983. doi:10.1038/ng2062
Haiman CA, Patterson N, Freedman ML, Myers SR, Pike MC, Waliszewska A, Neubauer J, Tandon A, Schirmer C, McDonald GJ, Greenway SC, Stram DO, Le Marchand L, Kolonel LN, Frasco M, Wong D, Pooler LC, Ardlie K, Oakley-Girvan I, Whittemore AS, Cooney KA, John EM, Ingles SA, Altshuler D, Henderson BE, Reich D (2007) Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet 39:638–644. doi:10.1038/ng2015
Hardy J, Singleton A (2009) Genomewide association studies and human disease. N Engl J Med 360:1759–1768. doi:10.1056/NEJMra0808700
Laird NM, Horvath S, Xu X (2000) Implementing a unified approach to family-based tests of association. Genet Epidemiol 19 Suppl 1: S36-42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M
Lange EM, Sun J, Lange LA, Zheng SL, Duggan D, Carpten JD, Gronberg H, Isaacs WB, Xu J, Chang BL (2008) Family-based samples can play an important role in genetic association studies. Cancer Epidemiol Biomarkers Prev 17:2208–2214. doi:10.1158/1055-9965
Lunetta KL, Faraone SV, Biederman J, Laird NM (2000) Family-based tests of association and linkage that use unaffected sibs, covariates, and interactions. Am J Hum Genet 66:605–614. doi:10.1086/302782
Manolio TA (2010) Genomewide association studies and assessment of the risk of disease. N Engl J Med 363:166–176. doi:10.1056/NEJMra0905980
Price AL, Zaitlen NA, Reich D, Patterson N (2010) New approaches to population stratification in genome-wide association studies. Nat Rev Genet 11:459–463. doi:10.1038/nrg2813
Salinas CA, Kwon E, Carlson CS, Koopmeiners JS, Feng Z, Karyadi DM, Ostrander EA, Stanford JL (2008) Multiple independent genetic variants in the 8q24 region are associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 17:1203–1213. doi:10.1158/1055-9965.EPI-07-2811
Schaid DJ, Chang BL (2005) Description of the International Consortium for Prostate Cancer Genetics, and failure to replicate linkage of hereditary prostate cancer to 20q13. Prostate 63:276–290. doi:10.1002/pros.20198
Sun J, Lange EM, Isaacs SD, Liu W, Wiley KE, Lange L, Gronberg H, Duggan D, Carpten JD, Walsh PC, Xu J, Chang BL, Isaacs WB, Zheng SL (2008a) Chromosome 8q24 risk variants in hereditary and non-hereditary prostate cancer patients. Prostate 68:489–497. doi:10.1002/pros.20695
Sun J, Zheng SL, Wiklund F, Isaacs SD, Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami HO, Wiley KE, Dimitrov L, Li T, Turner AR, Adams TS, Adolfsson J, Johansson JE, Lowey J, Trock BJ, Partin AW, Walsh PC, Trent JM, Duggan D, Carpten J, Chang BL, Gronberg H, Isaacs WB, Xu J (2008b) Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nat Genet 40:1153–1155. doi:10.1038/ng.214
Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ (2008) Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet 40:310–315. doi:10.1038/ng.91
Witte JS (2007) Multiple prostate cancer risk variants on 8q24. Nat Genet 39:579–580. doi:10.1038/ng0507-579
Xu J, Dimitrov L, Chang BL, Adams TS, Turner AR, Meyers DA, Eeles RA, Easton DF, Foulkes WD, Simard J, Giles GG, Hopper JL, Mahle L, Moller P, Bishop T, Evans C, Edwards S, Meitz J, Bullock S, Hope Q, Hsieh CL, Halpern J, Balise RN, Oakley-Girvan I, Whittemore AS, Ewing CM, Gielzak M, Isaacs SD, Walsh PC, Wiley KE, Isaacs WB, Thibodeau SN, McDonnell SK, Cunningham JM, Zarfas KE, Hebbring S, Schaid DJ, Friedrichsen DM, Deutsch K, Kolb S, Badzioch M, Jarvik GP, Janer M, Hood L, Ostrander EA, Stanford JL, Lange EM, Beebe-Dimmer JL, Mohai CE, Cooney KA, Ikonen T, Baffoe-Bonnie A, Fredriksson H, Matikainen MP, Tammela T, Bailey-Wilson J, Schleutker J, Maier C, Herkommer K, Hoegel JJ, Vogel W, Paiss T, Wiklund F, Emanuelsson M, Stenman E, Jonsson BA, Gronberg H, Camp NJ, Farnham J, Cannon-Albright LA, Seminara D (2005) A combined genomewide linkage scan of 1, 233 families for prostate cancer-susceptibility genes conducted by the international consortium for prostate cancer genetics. Am J Hum Genet 77:219–229. doi:10.1086/432377
Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G (2007) Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39:645–649. doi:10.1038/ng2022
Acknowledgment
We would like to express our gratitude to the many families who participated in the studies involved in the International Consortium for Prostate Cancer Genetics (ICPCG). The ICPCG, including the consortium’s Data Coordinating Center (DCC), is made possible by a grant from the National Institutes of Health U01 CA89600 (to William B. Isaacs). Additional support to participating groups, or members within groups, is as follows: University of Utah Group: The authors appreciate the support of the University of Utah Huntsman Cancer Institute (to Lisa A. Cannon-Albright). FHCRC/NHGRI Group: Partial support was provided by the Fred Hutchinson Cancer Research Center (to Janet L. Stanford) and National Human Genome Research Institute (to Elaine A. Ostrander). ACTANE Group: We appreciate the support of the CR-UK grant A8385 and the NIHR to the Biomedical Research Centre at The Institute of Cancer Research and Royal Marsden NHS Foundation Trust (to Ros Eeles), and Cancer Research UK (to Doug Easton). University of Umeå Group: Partial support was provided by the Swedish Cancer Society and a Spear grant from the Umeå University Hospital, Umeå, Sweden (to Henrik Grönberg). University of Tampere Group: We appreciate the support of the Competitive Research Funding of the Pirkanmaa Hospital District (9L091), Reino Lahtikari Foundation, Finnish Cancer Organisations, Sigrid Juselius Foundation and Academy of Finland (116437 and 126714) (to Johanna Schleutker). Northwestern University Group: Partial support was provided from Robert H. Lurie Comprehensive Cancer Center and the Urological Research Foundation (to William J. Catalona). University of Michigan Group: Partial support was provided by NIH P50 CA69568, NIH R01 CA79596 (to Kathleen Cooney), and the University of Michigan Comprehensive Cancer Center. Data Coordinating Center: Partial support was provided by NCI CA119069 and CA129684 (to Jianfeng Xu). We also thank other investigators who contributed to this work: ACTANE Group: Daniel Leongamornlert, Ed Saunders, Malgorzata Tymrakiewicz, Lynne O’Brien, Emma Sawyer, Rosemary Wilkinson, and Stephen Edwards from The Institute of Cancer Research, Sutton, Surrey. University of Ulm Group: Manuel Luedeke and Mark Schrader from Department of Urology, University of Ulm, Germany; Josef Hoegel and Christian Kubisch from Institute of Human Genetics, University of Ulm, Germany; and Kathleen Herkommer from Department of Urology, Technical University of Munich, Germany.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
G. Jin and L. Lu contributed equally to this work.
K.A. Cooney, A.M. Ray, K.A. Zuhlke and E.M. Lange are the members of the University of Michigan ICPCG Group.
L.M. FitzGerald and J.L. Stanford are the members of the FHCRC/NHGRI ICPCG Group.
W.D. Foulkes, G.G. Giles, J.L. Hopper, G. Severi, R. Eeles, D. Easton, Z. Kote-Jarai, and M. Guy are the members of the ACTANE Consortium ICPCG Group.
A. Rinckleb, C. Maier and W. Vogel are the members of the University of Ulm ICPCG Group.
S.N. Thibodeau, S.K. McDonnell and D.J. Schaid are the members of the Mayo Clinic ICPCG Group.
F. Wiklund, H. Grönberg and M. Emanuelsson are the members of the University of Umeå ICPCG Group.
A.S. Whittemore, I. Oakley-Girvan and C.-L. Hsieh are the members of the BC/CA/HI ICPCG Group.
T. Wahlfors, T. Tammela and J. Schleutker are the members of the University of Tampere ICPCG Group.
Rights and permissions
About this article
Cite this article
Jin, G., Lu, L., Cooney, K.A. et al. Validation of prostate cancer risk-related loci identified from genome-wide association studies using family-based association analysis: evidence from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum Genet 131, 1095–1103 (2012). https://doi.org/10.1007/s00439-011-1136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-011-1136-0