Abstract
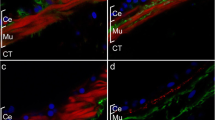
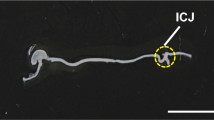
Holothurians (sea cucumbers) have been known from ancient times to have the capacity to regenerate their internal organs. In the species Holothuria glaberrima, intestinal regeneration involves the formation of thickenings along the free mesentery edge; these thickenings will later give rise to the regenerated organ. We have previously documented that a remodeling of the extracellular matrix and changes in the muscle layer occur during the formation of the intestinal primordium. In order to analyze these changes in depth, we have now used immunocytochemical techniques and transmission electron microscopy. Our results show a striking disorganization of the muscle layer together with myocyte dedifferentiation. This dedifferentiation involves nucleic activation, disruptions of intercellular junctions, and the disappearance of cell projections, but more prominently, the loss of the contractile apparatus by the formation and elimination of spindle-like structures. Muscle dedifferentiation can be seen as early as 2 days following evisceration and continues during the next 2 weeks of the regeneration process. Dedifferentiation of myocytes might result in cells that proliferate and give rise to new myocytes. Alternatively, dedifferentiating myocytes could give rise to cells with high nuclear-to-cytoplasmic ratios, with some being eliminated by apoptosis. Our results, together with those in other regeneration models, show that myocyte dedifferentiation is a common event in regeneration processes and that the dedifferentiated cells might play an important role in the formation of the new tissues or organs.







Similar content being viewed by others
References
Brockes JP, Kumar A (2002) Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev 3:566–574
Byrne M (1985) Evisceration behavior and the seasonal incidence of evisceration in the holothurian Eupentacta quinquesemita (Selenka). Ophelia 24:91–101
Byrne M (2001) The morphology of autotomy structures in the sea cucumber Eupentacta quinquesemita before and during evisceration. J Exp Biol 204:849–863
Candia-Carnevali MD, Bonasoro F (2001) Microscopic overview of crinoid regeneration. Microsc Res Tech 55:403–426
Candia-Carnevali MD, Bonasoro F, Patrono M, Thorndyke MC (1998) Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: the potencial of arm explants. Dev Genes Evol 208:421–430
Cornec JP, Cresp J, Delye P, Hoarau F, Reynaud G (1987) Tissue responses and organogenesis during regeneration in the oligochete Limnodrilus hoffmeisteri (Clap.). Can J Zool 65:403–414
Dolmatov IY (1992) Regeneration of aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuria, Dendrochitrota). Monogr Dev Biol 23:40–50
Dolmatov IY, Ginanova TT (2001) Muscle regeneration in holothurians. Microsc Res Tech 55:452–463
Dolmatov IY, Eliseikina MG, Bulgakov AA, Ginanova TT, Lamash NE, Korchagin VP (1996) Muscle regeneration in the holothurian Stichopus japonicus. Rouxs Arch Dev Biol 205:486–493
Echeverri K, Tanaka EM (2002) Mechanisms of muscle dedifferentiation during regeneration. Semin Cell Dev Biol 13:353–360
Echeverri K, Clarke JDW, Tanaka EM (2001) In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev Biol 236:151–164
Emson RH, Wilkie IC (1980) Fission and autotomy in echinoderms. Oceanogr Mar Biol Annu Rev 18:155–250
Feral JP, Massin C (1982) Digestive systems: Holothuroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 191–212
Fontes M, Coulon J, Delgrossi MH, Thouveny Y (1983) Muscle dedifferentiation and contractile protein synthesis during post-traumatic regeneration by Owenia fusiformis (polychaete annelid). Cell Differ 13:267–282
García-Arrarás JE, Greenberg MJ (2001) Visceral regeneration in holothurians. Microsc Res Tech 55:438–451
García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres II, Díaz-Miranda L, Torres-Avillán I (1998) Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J Exp Zool 281:288–304
García-Arrarás JE, Díaz-Miranda L, Torres-Vázquez I, File S, Jiménez L, Rivera-Bermudez K, Arroyo E, Cruz W (1999) Regeneration of the enteric nervous system in the sea cucumber Holothuria glaberrima. J Comp Neurol 406:461–475
García-Arrarás JE, Rojas-Soto M, Jiménez LB, Díaz-Miranda L (2001) The enteric nervous system of echinoderms: unexpected complexity revealed by neurochemical analysis. J Exp Biol 204:865–873
Hay ED (1959) Electron microscopic observations of muscle dedifferentiation in regeneration Amblystoma limbs. Dev Biol 1:555–585
Hyman L (1955) The invertebrates: Echinodermata. McGraw-Hill, New York
Kumar A, Velloso CP, Imokawa Y, Brockes JP (2000) Plasticity of retrovirus-labelled myotubes in the newt limb regeneration blastema. Dev Biol 218:125–136
Leibson NL (1992) Regeneration of digestive tube in holothurians Stichopus japonicus and Eupentacta fraudatrix. Monogr Dev Biol 23:51–61
Lo DC, Allen F, Brockes JP (1993) Reversal of muscle differentiation during urodele limb regeneration. Proc Natl Acad Sci USA 90:7230–7234
Mashanov VS, Dolmatov IY (2001) Regeneration of digestive tract in the pentactulae of the far-eastern holothurian Eupentacta fraudatrix (Holothuroidea: Dendrochirota). Invert Reprod Dev 39:143–151
Murray G, García-Arrarás JE (2004) Myogenesis during holothurian intestinal regeneration. Cell Tissue Res 318:515–524
Quiñones JL, Rosas R, Ruiz DC, García-Arrarás JE (2002) Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol 250:181–197
Rieger RM, Lombardi J (1987) Ultrastructure of the coelomic lining in echinoderm podia: significance for concepts in the evolution of muscle and peritoneal cells. Zoomorphology 107:191–208
Sánchez Alvarado A (2000) Regeneration in the metazoans: why does it happen? BioEssays 22:578–590
Smiley S (1994) Holothuroidea. In: Harrison F, Chia FS (eds) Microscopic anatomy of invertebrates, vol 14. Wiley Liss, New York, pp 401–471
Tanaka EM (2003) Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev 13:497–501
VandenSpiegel D, Jangoux M, Flammang P (2000) Maintaining the line of defense: regeneration of Cuvierian tubules in the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Biol Bull 198:34–49
Vetvicka V, Sima P, Cooper EL, Bilej M, Roch P (1994) Immunology of annelids. CRC Press, Boca Raton
Acknowledgements
We thank Griselle Valentin for technical assistance with the immunocyochemical experiments and Mr. Camillo Cangani for assistance with the transmission electron microscope.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NSF (IBN-0110692) and NIH-MBRS (S06GM08102). We also acknowledge partial support from RCMI (RRO-3641-01), the Department of Biology, and the University of Puerto Rico.
Rights and permissions
About this article
Cite this article
Candelaria, A.G., Murray, G., File, S.K. et al. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima . Cell Tissue Res 325, 55–65 (2006). https://doi.org/10.1007/s00441-006-0170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0170-z