Abstract
Microparticles are submicron vesicles shed from plasma membranes in response to cell activation, injury, and/or apoptosis. The measurement of the phospholipid content (mainly phosphatidylserine; PSer) of microparticles and the detection of proteins specific for the cells from which they are derived has allowed their quantification and characterization. Microparticles of various cellular origin (platelets, leukocytes, endothelial cells) are found in the plasma of healthy subjects, and their amount increases under pathological conditions. Endothelial microparticles (EMP) not only constitute an emerging marker of endothelial dysfunction, but are also considered to play a major biological role in inflammation, vascular injury, angiogenesis, and thrombosis. Although the mechanisms leading to their in vivo formation remain obscure, the release of EMP from cultured cells can be caused in vitro by a number of cytokines and apoptotic stimuli. Recent studies indicate that EMP are able to decrease nitric-oxide-dependent vasodilation, increase arterial stiffness, promote inflammation, and initiate thrombosis at their PSer-rich membrane, which highly co-expresses tissue factor. EMP are known to be elevated in acute coronary syndromes, in severe hypertension with end organ damage, and in thrombotic thrombocytopenic purpura, all conditions associated with endothelial injury and pro-thrombotic state. The release of EMP has also been associated with endothelial dysfunction of patients with multiple sclerosis and lupus anticoagulant. More recent studies have focused on the role of low shear stress leading to endothelial cell apoptosis and subsequent EMP release in end-stage renal disease. Improved knowledge of EMP composition, their biological effects, and the mechanisms leading to their clearance will probably open new therapeutic approaches in the treatment of atherothrombosis.
Similar content being viewed by others
Introduction
Maintenance of an intact monolayer endothelial cell barrier is crucial for normal vascular structure and function and exerts atheroprotective effects in vivo through the release of substances that promote anticoagulation, inhibit inflammation, and induce vasodilation. Activation or injury of the endothelium leads to a variety of inflammatory disorders including the release of microparticles (MP).
MP are defined as vesicles that are of less than 1 μm in diameter and that are shed from the plasma membranes of diverse cell types in response to activation, injury, and/or apoptosis (Hugel et al. 2005). As early as mid-last century, human serum was suspected to contain a subcellular platelet-like pro-coagulant factor (Chargaff and West 1946; O’Brien 1955). Electron-microscopic techniques allowed P. Wolf et al. to identify this subcellular factor as platelet-derived MP, first referred to as “platelet dust”, capable of facilitating thrombin generation in the same manner as that of intact platelets (Wolf 1967). Further in vitro studies provided evidence that activated platelets released MP during their attachment to the vascular wall (Warren and Vales 1972) opening the door to the more general comprehension of cell-derived MP formation and release. Circulating MP of different cellular origins, predominantly platelet-derived MP supporting low-grade hemostasis, are found in the plasma of healthy subjects (Berckmans et al. 2001; Leroyer et al. 2007). MP involvement in diseases was first demonstrated in idiopathic thrombotic thrombocytopenic purpura (TTP; Kahn et al. 1975). Since that time, the relevance of MP in various pathological conditions has been widely studied with respect to their pro-coagulant properties and their major roles in inflammation and vascular dysfunction.
The aim of this review is to illustrate, thanks to recent clinical and laboratory studies, the putative or demonstrated involvement of endothelial MP (EMP) in diseases, not only as prognostic markers, but also as biological agents and potential therapeutic targets.
EMP generation and release
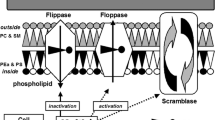
The knowledge of general molecular mechanisms leading to membrane cell vesiculation and MP liberation comes from in vitro studies on platelets and can be extended to other MP types (VanWijk et al. 2003; Hugel et al. 2005; Boulanger et al. 2006; Piccin et al. 2006). Briefly, resting cell membrane is characterized by its phospholipid distribution, with phosphatidylcholine and sphingomyelin located on its external layer, and phosphatidylethanolamine and phosphatidylserine (PSer) on its inner side. This resting phospolipid asymmetry is ensured by an active transmembrane enzymatic balance involving flippase, floppase, and scramblase equilibrium. Cell activation or apoptosis is associated with a prompt release of intracellular calcium by the endoplasmic reticulum (Fig. 1). The sudden increase in cytosolic calcium changes the transmembrane steady state, resulting in PSer externalization, and activates cytosolic enzymes including calpain, leading to the cleavage of cytoskeleton filaments. These phenomena result in the blebbing and shedding of membrane-derived MP into the extracellular fluid (Fig. 1).
Possible phathways leading to endothelial microparticles release. Adapted from Boulanger et al. (2006)
The precise mechanisms leading to in vivo MP generation by endothelial cells remain unclear. In vitro, a variety of prolonged stimuli are able to induce EMP vesiculation from cultured endothelial cells (Fig. 2). Combes et al. (1999) first described the generation of EMP from human umbilical endothelial cells stimulated by tumor necrosis factor α. However, EMP release can be triggered by non apoptotic stimuli as well (Sapet et al. 2006). Other pro-inflammatory, pro-thrombotic, pro-apotpotic, or oxidative substances have been shown to stimulate EMP release (Leroyer et al. 2008) (Fig. 2). Interestingly, endothelial NO (nitric oxide) synthase decoupling may participate, under certain conditions, to the production of EMP (Wang et al. 2007a, 2007b), emphasizing the possible reciprocal relationships between EMP and NO-dependent endothelial dysfunction. Of interest, our teams have demonstrated that EMP were increased in end-stage renal disease via uremic toxins (Faure et al. 2006) and via low shear stress responsible for EMP release (Boulanger et al. 2007) (Fig. 2).
Paracrine effects of endothelial microparticles (LPS lipopolysaccharide, LDL low-density lipoprotein, MP microparticles, NO nitric oxide). Adapted from Boulanger et al. (2006)
In addition to PSer, MP membranes express various phospholipids and oxidized lipids, plus diverse proteins specific for the cell type from which they originate. Moreover, the lipid and protein composition of MP depends on whether they are released by cell activation or by apoptotic stimulus (Jimenez et al. 2003). The special biochemical features of the MP surface have important consequences. First, PSer overexpression provides a common signature for the presence of MP. As PSer binds specifically to Annexin V, this marker is generally used to identify and quantify “total” MP amounts in blood or tissues. However, Annexin V is neither specific nor sensitive for MP. Annexin V may bind with other negatively charged phospholipids from cell fragments other than MP; inversely, numbers of MP have been shown to be Annexin-V-negative. Another consequence of PSer abundance is that it offers multiple binding sites for the coagulation factors II, Va, and Xa, thus providing MP pro-coagulant activity, together with the expression of tissue factor and the P-selectin/P-selectin glycoprotein ligand 1 system. A further consequence of the biochemical characteristics of a given MP membrane is that its lipid and protein composition may help in phenotyping the origin of the MP (cell type and MP release stimulus) and may explain their potential biological effects. In particular, in the case of EMP, the expression of numerous adhesion molecules provides (1) useful diagnostic tools for detecting them and (2) intriguing links for investigating their pathophysiology.
EMP characterization
In addition to PSer expression, which does not discriminate between MP parental cell types, EMP may express adhesion molecules specific for mature endothelial cells (Table 1): CD54 (ICAM-1: intercellular adhesion molecule 1), CD62E (E-selectin), CD62P (P-selectin), or CD31 (PECAM: platelet-endothelial cell adhesion molecule). Since CD31 is also expressed by platelet-derived MP, EMP specificity is ensured by the CD31+/CD41- phenotype (CD41 being the platelet integrin GPIIbIIIa). EMP also exhibit specific antigens such as CD105 (endoglin, a proliferation-associated protein), CD144 (VE-cadherin), and CD146 (S endo 1, an endothelial juntional protein), and they also bind von Willebrand factor (vWF).The variability of surface antigen expression as regards the MP release stimulus has been studied in vitro (Jimenez et al. 2003) and ex vivo (Abid-Hussein et al. 2003): apoptosis-induced EMP are more likely to express the constitutive endothelial cell marker PECAM (CD31), whereas activation-induced EMP increase their expression of inducible endothelial markers such as CD62E.
Thanks to these phenotypic characteristics, EMP can be identified in platelet-free plasma (supernatants from whole blood obtained after a 20-min centrifugation at 1550g followed by a 5-min centrifugation at 13,000g) by means of the following two main methods.
Solid-phase capture and enzyme-linked immunosorbent assay
After the capture of PSer+ MP by immobilized Annexin V, the addition of antibodies specific for endothelial antigens allows the sorting of EMP. The addition of pro-thrombinase complex factors (calcium, factor II, factor Xa, factor Va) leads to thrombin generation, the intensity of which is directly related to the amount of the limiting factor, PSer, reflecting the amount of MP. Therefore, solid-phase capture assay offers both qualitative and quantitative (pro-coagulant activity) information on MP content.
Flow cytometry analysis
MP were initially reported to be Annexin V+ on flow cytometry. The incubation of platelet-free plasma with additional markers specific to the endothelial cell lineage has allowed further characterization and analysis of EMP. With flow cytometry, MP are defined as events with a size of 0.2–1 μm. After gating with respect to this size population, they can easily be quantified by the number and density of events.
As mentioned above, the lack of specificity and sensibility of Annexin V binding may be a limitation for the accurate quantification of MP. Furthermore, the large panel of available markers for EMP characterization and the lack of a reference method (flow cytometry versus solid capture assay) makes it mandatory to standardize EMP measurement methods (Table 1). For example, clinical studies on the relationships between circulating EMP and atherosclerotic cardiovascular disease may not be comparable with others involving different methods of measurement. Moreover, recent advances in proteomics and lipidomics have demonstrated that the protein content of lymphocyte-derived MP membrane is highly influenced by the cell culture medium and the type of stimulus for MP generation (Miguet et al. 2006). Therefore, special caution should be taken when extrapolating laboratory experiments to the results of clinical studies, because a given MP type may be present with a variety of compositions and biological behavioral patterns in vitro and in vivo.
EMP in healthy conditions
Under normal conditions, vessel homeostasis is ensured by the anti-inflammatory, anti-thrombotic, and anti-atherogenic properties of the intact endothelial cell monolayer. Its functional integrity is maintained by low continuous cell regeneration and the incorporation of endothelial progenitor cells (EPC). The maintenance of laminar-fluid shear stress ensures endothelial cell survival and quiescence (Boulanger et al. 2007). Endothelial activation remains local, low-grade, and reversible. Subsequently, circulating EMP content remains poorly detectable (Berckmans et al. 2001; Leroyer et al. 2007). Protective low-grade pro-coagulant activity is maintained by circulating platelet-derived MP (Berckmans et al. 2001) and by EMP that express ultra-large vWF multimers, which increase platelet clot stability (Jy et al. 2005), in balance with anti-thrombotic proteins expressed at their surface (Perez-Casal et al. 2005).
EMP under pathological conditions
In the case of acute injury, endothelial cells may respond by dislodgement-induced apoptosis and EMP generation, loss of thrombomodulin, and loss of tissue factor inhibitor, leading to a pro-coagulant state (Bombeli et al. 1997). A variety of pathological conditions have been shown to involve EMP, the participation of which often remains unclear: is EMP vesiculation and release a cause or a consequence of the clinical disorders (Fig. 2)? Most of the time, EMP appear to be involved in a vicious circle together with, and amplified by, the deleterious effects of other biological effectors including MP of other origins. Three types of studies raise these questions. (1) In vivo study in humans consists of quantifying and characterizing circulating MP and showing associations with clinical manifestations or risk factors. (2) Ex vivo studies analyze the potential effects of MP isolated from blood or tissue of diverse types of patients. (3) Laboratory studies investigate the mechanisms of generation, behavior, properties of the MP produced in vitro from cultured cells.
Atherosclerotic disease
Atherosclerosis is promoted and perpetuated by an early and constant endothelial dysfunction. Given their biochemical composition, nature, and biological effects, EMP appear to be largely involved in atherogenesis (VanWijk et al. 2003). Indeed, they carry oxidized phospholipids at their surface, they express adhesion molecules, and they trigger the release of chemokines by endothelial cells, which in turn attract leukocytes to the endothelium. Moreover, in-vitro-generated EMP activate neutrophiles, initiate and propagate thrombin generation, and induce matrix degradation by exposing matrix metalloproteinase 2 and 9 (MMP) (Diamant et al. 2004). This is one of the explanations of the roles of EMP in angiogenesis and in plaque rupture (Leroyer et al. 2008).
Acute coronary syndromes
In 2000, Z. Mallat and coworkers showed significant increases of CD31+ and CD146+ EMP levels in the circulating blood of patients with acute coronary syndrome, as compared with stable coronary and non-coronary controls (Mallat et al. 2000). Their pro-coagulant potential was assessed by their Annexin-V binding, and their deleterious potential effects on endothelial function were further demonstrated ex vivo (Boulanger et al. 2001). Other studies of coronary heart disease have analyzed the relationships between EMP levels and the degree of morphological or functional coronary abnormalities and have found that the amount of circulating EMP is correlated positively with the extent and severity of coronary stenosis at angiography (Bernal-Mizrachi et al. 2003) and with the coronary endothelium-dependent dysfunction, as measured by the vasodilator response following acetylcholine infusion (Koga et al. 2005).
Stroke and peripheral arterial disease
The involvement of platelet MP, which reflect platelet activation leading to vaso-occlusive events, has been shown in patients with cerebrovascular disease or multi-infarct dementia (Lee et al. 1993). In contrast, one study has demonstrated the lack of usefulness of CD31+ or CD62E+ EMP for discrimating subjects who have had ischemic stroke, as documented by magnetic resonance imaging, from those who have not (Williams et al. 2007). So far, although peripheral arterial disease might also involve EMP, only the roles of platelet-derived MP have been showed in association with mural thrombus formation of aortic aneurysm (Piccin et al. 2006)
Silent atherosclerosis
Of utmost interest in clinical practice is the detection of high-cardiovascular risk subjects in order to be able to treat them intensively with risk-reduction therapies. In this field, together with cardiovascular risk factors assessment, non-invasive subclinical atherosclerosis detection provides useful predictive tools (Simon et al. 2006, 2007). Some studies have analyzed the relationships between circulating MP and structural or functional early alterations of the arterial wall.
EMP and endothelial dysfunction
Endothelial function can be assessed early on and non-invasively in apparently healthy subjects by calculating brachial artery flow-mediated vasodilation (Chironi et al. 2008), a surrogate of in vivo NO release in response to an increase in acute shear stress. Similarly to the relationship of EMP with coronary endothelial dysfunction (Koga et al. 2005), altered flow-mediated vasodilation of patients with renal insufficiency has been demonstrated to be associated with high levels of MP of endothelial origin (CD144+; Amabile et al. 2005). Similar results have be found in diabetic men (Esposito et al. 2007) and in obese women (Esposito et al. 2006). Ex vivo studies provide evidence that MP isolated from the blood of patients with acute coronary syndrome can cause severe endothelial dysfunction in rat aorta by altering the endothelial NO transduction pathway, but not endothelial NO synthase expression (Boulanger et al. 2001). These clinical and laboratory observations demonstrate that the higher the circulating EMP levels, the worse the endothelium-dependent vasodilation, offering EMP a useful significance as endothelial dysfunction markers.
EMP and arterial stiffness
Arterial stiffness, the sclerotic part of atherosclerosis, corresponds to the loss of arterial compliance. It can be assessed non-invasively by measuring carotid-femoral pulse wave velocity and provides predictive value regarding the risk of future cardiovascular events (Simon et al. 2006, 2007). Again, in vivo clinical studies highlight the relationships between circulating levels of EMP and arterial stiffness in patients with end-stage renal failure (Amabile et al. 2005) and in healthy subjects (Wang et al. 2007a, 2007b), independently of age and blood pressure.
EMP and silent plaque
In a study in 216 asymptomatic subjects with cardiovascular risk factors, we showed that plaque presence and diffusion was related to leukocyte-derived CD11a+ circulating MP, but with no association with EMP levels (Chironi et al. 2006). This lack of relationship, contrasting with the evidence of endothelial dysfunction in silent atherosclerosis, can be explained by the different technique of measurement from that in other studies (Table 1). Moreover, carotid plaques, which are rich in leukocyte-derived MP (Mallat et al. 1999; Leroyer et al. 2007), may release MP into the circulating blood and mask the detection of other MP types.
Cardiovascular risk factors
Metabolic syndrome and diabetes
Metabolic syndrome is a well-known condition providing an underlying pro-inflammatory, pro-atherogenic, and pro-thrombotic state. Conversely, circulating EMP were found to be increased in 33 patients with metabolic syndrome and favored ex vivo platelet and leukocyte activation (Arteaga et al. 2006). In this study, CD31+ EMP were markedly elevated, whereas CD62E+ EMP remained at normal levels, suggesting apoptotic stimuli rather than endothelial cell activation (Arteaga et al. 2006). Intriguingly, concordant studies did not find EMP to be increased in type 2 diabetes mellitus (Diamant et al. 2002; Sabatier et al. 2002), whereas elevated levels of EMP were associated with microalbuminuria in patients with type 1 diabetes (Sabatier et al. 2002).
Hypertension
Preston et al. (2003) found a strong increase in EMP from patients with severe hypertension, as compared with mild hypertensive and normotensive subjects. Even after adjustment for concomitant risk factors, EMP levels were highly correlated with systolic and diastolic blood pressure, suggesting that EMP could play a pathogenic role in mediating target organ injury in severe hypertension (Preston et al. 2003).
Smoking
Active smoking is a well-established atherosclerosis risk factor, together with a cause of endothelial dysfunction and alteration of EPC. Secondhand smoking in healthy subjects has recently been shown to provoke a sustained increase in EMP and dysfunctional EPC, very rapidly after acute endothelial dysfunction (Heiss et al. 2008).
Deep venous thrombosis and pulmonary embolism
Despite their theoretical involvement in pro-coagulant mechanisms leading to veinous thromboembolism, EMP studies rarely include these pathological conditions (Piccin et al. 2006). Nevertheless, the predominant role of platelet-derived MP may mask that of EMP, mainly because thrombosis in these situations is more patent than in endothelial damage.
Hematological diseases
Sickle cell disease
This genetic hemoglobinopathy leads to falciformation of erythrocytes and subsequent hemolysis and thrombotic events. It is characterized by the alternance of chronic and acute phases. The chronic phase is associated with a pro-coagulant and pro-inflammatory state with subclinical endothelial damage and consequent detachment of endothelial cells, leading to low-level EMP generation (Shet et al. 2003). Typically, EMP described in chronic sickle cell disease strongly express CD105 (ICAM-1), closely related with hemolysis markers (Shet et al. 2003). Thus, the measurement of CD105+ EMP levels during the chronic phase of sickle cell disease may provide a useful tool for predicting hemolysis severity and for managing specific therapies. During crises, together with erythrocyte, monocyte, and platelet MP, EMP levels dramatically increase, with the EMP highly expressing tissue factor leading to possible severe thrombotic events.
Thrombotic thrombocytopenic purpura
TTP is characterized by failure to cleave extremely large multimers of vWF in the circulation. Under normal conditions, these highly pro-thrombotic multimers, released from the endothelial Weibel-Palade bodies, are rapidly proteolysed by a plasma metalloprotease. Congenital or acquired quantitative or qualitative deficiencies of this protease lead to TTP, with the formation of large platelet aggregates bound by vWF. In addition to increased levels of platelet MP in acute and chronic phase of TTP, the role of EMP has emerged from in vivo and in vitro studies. Indeed, circulating EMP levels increase long before the onset of patent thrombocytopenia (Jimenez et al. 2001) and may therefore provide an early prognostic marker in chronic TTP. The roles of EMP consist of facilitating cell adhesion and platelet aggregation, as suggested by the 13-fold increase in adhesion molecules ICAM-1 and vascular cell adhesion molecule 1 in TTP (Jimenez et al. 2001), together with EMP enrichment of surface vWF (Jy et al. 2005).
Paroxysmal nocturnal hemoglobinuria
This rare hematological syndrome is characterized by a complement-mediated erythrocyte destruction leading to hemolysis. Its clinical features include the progression to aplastic anemia and a high prevalence of venous thrombosis. The latter is partly explained by MP pro-coagulant activity and chronic endothelial activation, as reflected by the high levels of circulating ICAM1+, CD144+ and CD105+ EMP (Simak et al. 2004).
Systemic diseases
Increased levels of circulating MPs have been observed in a number of systemic diseases, reflecting chronic underlying vascular endothelial damage and activation. EMP has been found to be elevated in systemic lupus with antiphospholipid syndrome (Combes et al. 1999; Dignat-George et al. 2004), in young subjects suffering medium and small vessel vasculitis (Brogan et al. 2004), in multiple sclerosis (Minagar et al. 2001), and in Crohn disease (Chamouard et al. 2005). EMP are also involved in septic patients, together with platelet and leukocyte MP (Soriano et al. 2005). The extent to which they contribute to the sepsis process and its complications is not known, but some evidence suggests that the presence of EMP and MP-cell conjugates predict a favorable outcome in severe sepsis (Soriano et al. 2005).
Heart failure
Heart failure can also be considered a systemic disease because it affects peripheral systemic vasculature, results in impaired endothelial function, and provides systemic release of inflammatory cytokines. The contribution of EMP in this condition is demonstrated by the findings of raised apoptotic EMP together with elevated inflammatory cytokine in patients with heart failure as compared with controls; this apopotosis is reversible on treatment with carvedilol (Rössig et al. 2000) or with vitamin C (Rössig et al. 2001).
Pulmonary arterial hypertension
Two recent studies provide consistent evidence that circulating EMP are increased in pulmonary arterial hypertension, and that their level is highly predictive of hemodynamic severity (Amabile et al. 2008; Bakouloula et al. 2008). Of note, consistent findings result from the two methods of EMP quantification (flow cytometry versus solid capture assay) with different EMP markers (Table 1). These results suggest that pulmonary arterial hypertension is associated with intense endothelial damage, the gravity of which might be monitored by assessing EMP amount.
End-stage renal disease with anemia
As previously mentioned, end-stage renal disease offers an important clinical model for studying EMP involvement. In brief, EMP have been shown to be increased in patients with severe kidney failure undergoing hemodialysis and to be correlated with endothelial dysfunction and arterial stiffness (Amabile et al. 2005). In addition to uremic toxins (Faure et al. 2006), the decreased whole blood viscosity and low shear stress involved in renal disease-related anemia contributes to endothelial apoptosis and EMP release (Boulanger et al. 2007).
Concluding remarks
Circulating EMP in human clearly constitute a marker of vascular disease and endothelial dysfunction in atherosclerosis, in haematological and in systemic inflammatory diseases. Given their pro-coagulant and pro-inflammatory properties, they probably support a large part of athero-thrombotic risk in clinically patent diseases. However, they also probably increase this risk in asymptomatic subjects, providing potential predictive value to be confirmed in prospective studies.
EMP, like other cell-derived MP, are huge biological effectors affecting various levels of cardiovascular physiopathology. They may initiate atherosclerosis by promoting endothelial dysfunction and arterial wall inflammation, and they also may contribute to plaque progression and rupture. New physiopathologic insights in this field should allow research into therapies for modulating their effects. In addition, a more precise description of their mechanisms of release and clearance might provide new therapeutic targets for cardiovascular risk reduction.
References
Abid-Hussein MN, Meesters EW, Osmanovic N, Romijn FP, Nieewland R, Sturk A (2003) Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo. J Thromb Haemost 1:2434–2443
Amabile N, Guérin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM (2005) Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16:3381–3388
Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, DeMarco T, Yeghiazarians Y (2008) Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med 177:1268–1275
Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, deMarchena E, Ahn YS (2006) Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 98:70–74
Bakouloula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, Desprez D, Chabot F, Weitzenblum E, Freyssinet JM, Chaouat A, Toti F (2008) Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med 177:536–543
Berckmans RJ, Nieuwlands R, Boing AN, Romijn FP, Hack CE, Sturk A (2001) Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 85:639–646
Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, deMarchena E, Ahn YS (2003) High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 145:962–970
Bombeli T, Karsan A, Tait JF, Harlan JM (1997) Apoptotic vascular endothelial cells become procoagulant. Blood 89:2429–2442
Boulanger C, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z (2001) Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104:2649–2652
Boulanger C, Amabile N, Tedgui A (2006) Circulating microparticles. A potential prognostic marker for atherosclerotic vascular disease. Hypertension 48:180–186
Boulanger C, Amabile N, Guérin AP, Pannier B, Leroyer AS, Nguyen C, Mallat Z, Tedgui A, London G (2007) In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension 49:1–7
Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N, Dillon MJ (2004) Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 50:927–936
Chamouard P, Desprez D, Hugel B, Kunzelmann C, Gidon-Jeangirard C, Lessard M, Baumann R, Freyssinet JM, Grunebaum L (2005) Circulating cell-derived microparticles in Crohn’s disease. Dig Dis Sci 50:574–580
Chargaff E, West R (1946) The biological significance of the thromboplastic protein of blood. J Biol Chem 166:189–197
Chironi G, Simon A, Hugel B, Del-Pino M, Gariepy J, Freyssinet JM, Tedgui A (2006) Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol 26:2775–2780
Chironi G, Craiem D, Miranda-Lacet J, Levenson J, Simon A (2008) Impact of shear stimulus, risk factors burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. J Hypertens 26:508–515
Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F (1999) In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 104:93–102
Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JWA, Radder JK (2002) Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 106:2442–2447
Diamant M, Tushuizen ME, Sturk A, Nieuwland R (2004) Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest 34:392–401
Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, Veit V, Combes V, Gentile S, Moal V, Sanmarco M, Sampol J (2004) Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost 91:667–673
Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Giannetti G, Giugliano D (2006) Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab 91:3676–3679
Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Beneduce F, Rispoli M, De-Sio M, Giugliano D (2007) Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res 19:161–166
Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F (2006) Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 4:566–573
Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y (2008) Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol 51:1760–1771
Hugel B, Martinez C, Kunzelmann C, Freyssinet JM (2005) Membrane microparticles: two sides of the coin. Physiology 20:22–27
Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn ZS (2001) Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol 112:81–90
Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS (2003) Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 109:175–180
Jy W, Jimenez JJ, Mauro LM, Horstman LL, Cheng P, Ahn ER, Bidot CJ, Ahn YS (2005) Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. Thromb Haemost 3:1301–1308
Kahn I, Zucker-Franklin D, Karpatkin S (1975) Microthrombocytis and platelet fragmentation associated with idiopathic/autoimmune thrombocytopenic purpura. Br J Haematol 31:449–460
Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H (2005) Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 45:1622–1630
Lee YJ, Jy W, Horstman LL, Janania J, Reyes Y, Kelley RE, Ahn YS (1993) Elevated platelet microparticles in transient ischemic attacks, lacunar infarcts, and multi-infarct dementias. Thromb Res 72:295–304
Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM (2007) Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol 49:772–777
Leroyer AS, Tedgui A, Boulanger CM (2008) Role of microparticles in atherothrombosis. J Int Med 263:528–537
Mallat Z, Hugel B, Ohan J, Lesèche G, Freyssinet J, Tedgui A (1999) Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques. A role for apoptosis in plaque thrombogenicity. Circulation 99:348–353
Mallat Z, Benamer H, Hugel B, Benessiano J, Steg P, Freyssinet J, Tedgui A (2000) Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 101:841–843
Miguet L, Pacaud K, Felden C, Hugel B, Martinez MC, Freyssinet JM, Herbrecht R, Potier N, van Dorsselaer A, Mauvieux L (2006) Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics 6:153–171
Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Horstman LL, Ahn YS (2001) Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 56:1319–1324
O’Brien J (1955) The platelet-like activity of serum. Br J Haematol 1:223–228
Perez-Casal M, Downe C, Fukudome K, Marx G, Toh CH (2005) Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood 105:1515–1522
Piccin A, Murphy W, Smith O (2006) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21:157–171
Preston R, Jy W, Jimenez J, Mauro L, Horstman L, Valle M, Aime G, Ahn Y (2003) Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41:211–217
Rössig L, Haendeler J, Mallat Z, Hugel B, Freyssinet JM, Tedgui A, Dimmeler S, Zeiher AM (2000) Congestive heart failure induces endothelial cell apoptosis: protective role of carvedilol. J Am Coll Cardiol 36:2081–2089
Rössig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, Dimmeler S (2001) Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation 104:218–2187
Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Olivier C, Sampol J, Dignat-George F (2002) Type 1 and type 2 diabetic patients display different patterns of cellular MP. Diabetes 5:2840–2845
Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F (2006) Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108:1868–1876
Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP (2003) Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 102:2678–2683
Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG (2004) Elevatd circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol 125:804–813
Simon A, Chironi G, Levenson J (2006) Performance of subclinical arterial disease detection as a screening test for coronary heart disease. Hypertension 48:392–396
Simon A, Chironi G, Levenson J (2007) Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. Eur Heart J 28:2967–2971
Soriano AO, Jy W, Chirinos JA, Valdivia MA, Velasquez HS, Jimenez JJ, Horstman LL, Kett DH, Schein RM, Ahn YS (2005) Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit Care Med 33:2540–2546
VanWijk M, VanBavel E, Sturk A, Nieuwland R (2003) Microparticles in cardiovascular diseases. Cardiovasc Res 59:277–287
Wang JM, Huang YJ, Wang Y, Xu MG, Wang LC, Wang SM, Tao J (2007a) Increased circulating CD31+/CD42- microparticles are associated with impaired systemic artery elasticity in healthy subjects. Am J Hypertens 20:965–966
Wang JM, Wang Y, Huang JY, Yang Z, Chen L, Wang LC (2007b) Reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formation. J Vasc Res 44:241–248
Warren B, Vales O (1972) The release of vesicles from platelets following adhesion to vessel walls in vitro. Br J Exp Pathol 53:206–215
Williams JB, Jauch EC, Lindsell CJ, Campos B (2007) Endothelial microparticle levels are similar in acute ischemic stroke and stroke mimics due to activation and not apoptosis/necrosis. Acad Emerg Med 14:685–690
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from the Agence Nationale de la Recherche (Projet MIPRA-Met, ANR-05-PCOD-24–01).
Rights and permissions
About this article
Cite this article
Chironi, G.N., Boulanger, C.M., Simon, A. et al. Endothelial microparticles in diseases. Cell Tissue Res 335, 143–151 (2009). https://doi.org/10.1007/s00441-008-0710-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0710-9