Abstract
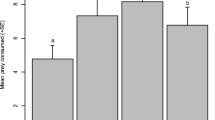
We explored whether a variation in predation and habitat complexity between conspecific populations can drive qualitatively different numerical dynamics in those populations. We considered two disjunct populations of the least killifish, Heterandria formosa, that exhibit long-term differences in density, top fish predator species, and dominant aquatic vegetation. Monthly censuses over a 3-year period found that in the higher density population, changes in H. formosa density exhibited a strong negative autocorrelation structure: increases (decreases) at one census tended to be followed by decreases (increases) at the next one. However, no such correlation was present in the lower density population. Monthly census data also revealed that predators, especially Lepomis sp., were considerably more abundant at the site with lower H. formosa densities. Experimental studies showed that the predation by Lepomis gulosus occurred at a much higher rate than predation by two other fish and two dragonfly species, although L. gulosus and L. punctatus had similar predation rates when the amount of vegetative cover was high. The most effective predator, L. gulosus, did not discriminate among life stages (males, females, and juveniles) of H. formosa. Increased predation rates by L. gulosus could keep H. formosa low in one population, thereby eliminating strong negative density-dependent regulation. In support of this, changes in H. formosa density were positively correlated with changes in vegetative cover for the population with a history of lower density, but not for the population with a history of higher density. Our results are consistent with the hypothesis that the observed differences among natural populations in numerical abundance and dynamics are caused in part by the differences in habitat complexity and the predator community.





Similar content being viewed by others
References
Almany GR (2004) Does increased habitat complexity reduce predation and competition in coral reef fish assemblages? Oikos 106:275–284
Baer CF (1998) Population structure in a south-eastern US freshwater fish, Heterandria formosa. II. Gene flow and biogeography within the St. Johns River drainage. Heredity 81:404–411
Beukers JS, Jones GP (1997) Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114:50–59
Box GEP, Jenkins GM, Reinsel GC (1994) Time series analysis, forecasting and control, 3rd edn. Prentice-Hall, Englewood Cliffs
Bradshaw W, Holzapfel C (1989) Life-historical consequences of density-dependent selection in the pitcher plant mosquito, Wyeomia smithii. Am Nat 133:869–887
Charlesworth B (1994) Evolution in age-structured populations. Cambridge University Press, Cambridge
Chatfield C (1996) The analysis of time series, an introduction. Chapman and Hall/CRC Press, Boca Raton
Cooper G (2001) Must there be a balance of nature? Biol Philos 16:481–506
De Roos AM, Persson L (2002) Size-dependent life-history traits promote catastrophic collapses of top predators. Proc Natl Acad Sci 99:12907–12912
De Roos AM, Persson L, McCauley E (2003a) The influence of size-dependent life-history traits on the structure and dynamics of populations and communities. Ecol Lett 6:473–487
De Roos AM, Persson L, Thieme HR (2003b) Emergent Allee effects in top predators feeding on structured prey populations. Proc R Soc Lond B 270:611–618
Dionne M, Folt CL (1991) An experimental analysis of macrophyte growth forms as fish foraging habitat. Can J Fish Aquat Sci 48:123–131
Ellner S, Turchin P (1995) Chaos in a noisy world-new methods and evidence from time-series analysis. Am Nat 145:343–375
Flynn AJ, Ritz DA (1999) Effect of habitat complexity and predatory style on the capture success of fish feeding on aggregated prey. J Mar Biol Assoc UK 79:487–494
Gunzburger MS, Travis J (2004) Evaluating predation pressure on green treefrog larvae across a habitat gradient. Oecologia 140:422–429
Hassell MP, Lawton JH, May RM (1976) Patterns of dynamical behaviour in single-species populations. J Anim Ecol 45:471–486
Jordon F (2002) Field and laboratory evaluation of habitat use by rainwater killifish (Lucania parva) in the St. Johns River Estuary, Florida. Estuaries 25:291–298
Kendall BE, Prendergast J, Bjornstad ON (1998) The macroecology of population dynamics: taxonomic and biogeographic patterns in population cycles. Ecol Lett 1:160–164
Law R, Bradshaw A, Putwain P (1977) Life-history variation in Poa annua. Evolution 31:233–246
Leips J, Travis J (1999) The comparative expression of life-history traits and its relationship to the numerical dynamics of four populations of least killifish. J Anim Ecol 68:595–616
Leips J, Travis J, Rodd FH (2000) Genetic influences on experimental population dynamics of the least killifish. Ecol Monogr 70:289–309
Mueller LD (1997) Theoretical and empirical examination of density-dependent selection. Annu Rev Ecol Syst 28:269–288
Ohgushi T (1995) Adaptive behavior produces population stability in herbivorous lady beetle populations. In: Cappuccino N, Price P (eds) Population dynamics: new approaches and synthesis. Academic, San Diego, pp 303–320
Pankratz A (1991) Forecasting with dynamic regression models. Wiley, New York
Persson L, Eklov P (1995) Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 76:70–81
Persson L, Leonardsson K, De Roos AM, Gyllenberg M, Christensen B (1998) Ontogenetic scaling of foraging rates and the dynamics of a size-structured consumer-resource model. Theor Popul Biol 54:270–293
Post JR, Parkinson EA, Johnston NT (1999) Density-dependent processes in structured fish populations: interaction strengths in whole-lake experiments. Ecol Monogr 69:155–175
Primack RB, Antonovics J (1982) Experimental ecological genetics in plantago. 7. Reproductive effort in populations of Plantago lanceolata L. Evolution 36:742–752
Rossiter M (1995) Impact of life-history evolution on population dynamics: predicting the presence of maternal effects. In: Cappuccino N, Price P (eds) Population dynamics: new approaches and synthesis. Academic, San Diego, pp 251–275
Sale PF, Tolimieri N (2000) Density dependence at some time and place? Oecologia 124:166–171
Sinclair ARE (1989) Population regulation in animals. In: Cherret JM (ed) Ecological concepts, the contribution of ecology to an understanding of the natural world. In: 29th symposium of the British Ecological Society. Blackwell, Oxford, pp 197–241
Soucy S, Travis J (2003) Multiple paternity and population genetic structure in natural populations of the poeciliid fish, Heterandria formosa. J Evol Biol 16:1328–1336
Travis J, Farr JA, Henrich S, Cheong RT (1987) Testing theories of clutch overlap with the reproductive ecology of Heterandria formosa. Ecology 68:611–623
Acknowledgements
This work was supported by National Science Foundation award DEB 99-03925 to JT. M. Richards and several anonymous reviewers provided helpful feedback on previous versions of the manuscript. We thank M. Aresco, R. Fuller, L. Horth, and B. Shoplock for help with the field censuses and M. Aresco for help with the predation experiments. We are grateful to K. Graffius-Ashcraft for facilities support for the predation experiments. This work was performed under Florida State University’s ACUC Protocol 9321 and complies with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Post
Rights and permissions
About this article
Cite this article
Richardson, J.M.L., Gunzburger, M.S. & Travis, J. Variation in predation pressure as a mechanism underlying differences in numerical abundance between populations of the poeciliid fish Heterandria formosa . Oecologia 147, 596–605 (2006). https://doi.org/10.1007/s00442-005-0306-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0306-y